Antarctica does Acid
Antarctica does Acid
Click for big version
Sea Acidification Model 2009
An idea that has been around since the 1980s has been confirmed by experiments and careful modeling based on recent measurements of the global levels of carbon dioxide: the 'greenhouse' gas. The oceans are becoming acidic. This is a serious threat to marine ecosystems, especially the Southern Ocean. The animals that make up the bottom of the food chain may disappear from the Southern Ocean, which will make big changes to the Antarctic ecosystem up to, and including, whales.
Gases in our atmosphere exist in equilibrium with the oceans, following a physical rule known as 'Henry's Law'. The amount of carbon dioxide in the atmosphere is in proportion to the amount of carbon dioxide that is dissolved in water. In pre-industrial times ocean life generated excess carbon dioxide which then was released into the atmosphere to reach a balance.
Now this has changed. With the increasing levels of carbon dioxide emitted by our fossil fuel burning society, more carbon dioxide is entering the ocean than is leaving it. The ocean absorbs about one million tonnes of carbon dioxide every hour, which is about ten times the natural pre-industrial rate. This carbon dioxide 'sink' into the ocean is the 'buffer' that has slowed the rate of global warming. It is estimated that the oceans have absorbed about 30% of the carbon dioxide released by our industrial society. In the next 50 years or so, the oceans will be absorbing about 80% of the carbon generated by human society, especially if no changes are made to reduce our generation of carbon dioxide.
This comes at a price. When carbon dioxide gas dissolves into seawater, it makes the water more acidic. At the same time, the increasing concentration of dissolved carbon dioxide reduces the concentration of dissolved calcium carbonate that is available to marine life in the ocean.
What does this mean?
If you are small and float in the ocean, a way to protect yourself is by having a shell. Just like corals, most of these small animals make their shells out of calcium carbonate. When they die these shells fall to the bottom. In England, the famous White Cliffs of Dover are made from these shells, which gives you an idea of how many of these animals live and die in the sea. In the ocean, these little animals are among the most important part of the ecosystem. They make up a critical part of the food chain. Other small animals eat them, then small or baby fish eat those animals, then other predators eat these small or baby fish, and so on.
With increased acidity two things happen; there is less carbonate available so these animals can't make their shells easily, and then the increased acidity erodes and weakens these shells by making them more brittle.
So there's less carbonate; what's the catch?
In a similar way to the balance of carbon dioxide between air and water, the sea maintains a physical balance of calcium carbonate. In the past the oceans were 'saturated' with calcium carbonate and marine organisms had an abundance of calcium carbonate to use. Coral reefs grew strong and big and the enormous blooms (sometimes thousands of square kilometres) of the small planktonic floating animals could make their shells.
As the levels of carbon dioxide in the seawater change, the point where the oceans are saturated with carbonate changes and the water becomes under-saturated; the amounts of calcium carbonate available to animals is not enough. When this happens, animals that use calcium carbonate can't make their shells very well, if they can make their shells at all. So they disappear.
The danger for the Southern Ocean
Recent experimental evidence and careful modeling show that polar regions will be the first and worst affected by this change. In past years, the belief was that a change in calcium carbonate levels would take hundreds of years to appear, but recent work shows that ecologically destructive changes can happen in decades- between 2050 and 2099. It sounds like a long time away, but your children will live in this new world. In the temperate and tropical parts of the World, calcium carbonate is abundant. Things are different in the Southern Ocean.
In the Southern Ocean, because of ocean dynamics, only one form of calcium carbonate is readily available for small marine animals. This is 'aragonite', and Antarctic animals rely on it. One of the most abundant animals is the pteropod, a tiny type of mollusk, or snail. It is at the bottom of the food chain and many animals, depend on it as food. Current research shows that by 2099, aragonite will not be available for pteropods to make their shells. They are likely to disappear.
So they're gone, so what?
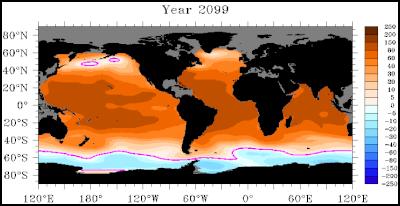
As a foundation of the food chain, a loss of pteropods will affect all other life. The abundance or availability of fish will change, which will affect animals like other fish, penguins, and seals. The abundance of krill will change, altering local ecosystems that rely on them for food and affect whales that migrate to feed in Antarctica. The attached picture tells the story: Where it is blue, there is not enough aragonite for the small animals, the pteropods, to live and grow shells. They will vanish. This picture is the middle, or median, estimate of ten models that were calculated. These ten models ranged from minimum additional levels of industrial carbon dioxide into the ocean; based on the most minor of climate change, to the highest climate change estimates.
Is this the Future?
In the 1980s, Dr Richard Feely wrote about the effects of altered carbon dioxide on seawater chemistry. These chemical effects are well known and measurable and it is basic science. Now we have good measurements and trends that we can fit with the ecosystems and changes. In the Southern Ocean aragonite is what used by key species in the food chain. It is difficult to extrapolate what changes might happen when they go, but the changes will be wide-ranging and profound and will happen in your, or your child's, lifetime. Researchers have run experiments where they have watched pteropod shells dissolve when the seawater gets acidic at levels of carbon dioxide that will be reached in fifty years. The problem with global warming is that it's a growth game. The earlier we make cuts to greenhouse gases the less reductions we have to make. If our society continues on as usual, the bigger the reduction in carbon dioxide our children have to make. It is a feedback. The less we do now the more we have to do later. This is also seen in the response: Just a few years ago, scientists were estimating the melting of the Antarctic ice sheets in the thousands of years, now they say it is a few hundred.
A certain thing
Most marine scientists are concerned. The acid ocean will alter coral reefs and change ocean ecosystems. This is known and has been documented in experiments. The change to the Southern Ocean will, by all indications, be devastating and fundamental.
More
information:
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?
cmd=Retrieve&db=PubMed&list_uids=16193043&dopt=Abstract
http://www.research.noaa.gov/spotlite/archive/spot_gcc.html
http://www2.cnrs.fr/en/377.htm?debut=48
http://www.precaution.org/lib/06/ocean_acidification_from_c02_060301.pdf
ENDS


 ASB Bank: ASB Business Survey - The Impact Of Trump's Tariffs, According To Kiwi Businesses
ASB Bank: ASB Business Survey - The Impact Of Trump's Tariffs, According To Kiwi Businesses University of Auckland: Will Robots Help Older People Stay Sharp?
University of Auckland: Will Robots Help Older People Stay Sharp? Electricity Authority: Authority Confirms New Next-Gen Switching Service; Proposes Multiple Trading Relationships For Consumers
Electricity Authority: Authority Confirms New Next-Gen Switching Service; Proposes Multiple Trading Relationships For Consumers Mānuka Charitable Trust: Mānuka Charitable Trust Warns Global Buyers Of Misleading Australian Honey Claims
Mānuka Charitable Trust: Mānuka Charitable Trust Warns Global Buyers Of Misleading Australian Honey Claims  Engineering New Zealand: NZ Building System Needs Urgent Improvement
Engineering New Zealand: NZ Building System Needs Urgent Improvement GNS Science: Bioshields Could Help Slow Tsunami Flow
GNS Science: Bioshields Could Help Slow Tsunami Flow


