MGR IV: Novartis Vaccines Enters The MeNZB Debate
Meningococcal Gold Rush IV:
Parents Welcome
Novartis Vaccines’ Entry into MeNZB Debate
By Ron Law, Risk & Policy Analyst
&
Barbara Sumner Burstyn, Writer/Researcher
Click
Here for earlier parts of this
series…
Throughout the debate surrounding the controversial MeNZB vaccine, Chiron (now called Novartis Vaccines) has remained strangely silent. On 14 November 2006 they broke that silence in a press release to criticize MeNZB vaccine researchers Ron Law and Barbara Sumner Burstyn. [1]
Law and Sumner Burstyn had publicized that Novartis have dumped the MeNZB vaccine technology in favour of trialing a genetically modified Meningococcal B vaccine. [2]
Novartis took exception to Law and Sumner Burstyn NOT referring to their August 2006 press statement that implied the shift to a genetically modified vaccine was not unexpected.
However, nearly two years ago in their first edition of the Meningococcal Gold Rush series, the authors stated that the experimental MeNZB vaccine was an orphan vaccine looking for a market. Law & Sumner Burstyn noted then Chiron planned to replace the MeNZB technology with a genetically engineered meningococcal B vaccine in the foreseeable future.
The bottom line is Novartis Vaccines have indeed dumped the MeNZB vaccine and technology as noted in our recent press release on 13 November 2006. [3]
Further to this opening shot, Novartis Vaccines makes a number of claims that need addressing.
1. No efficacy studies have ever been undertaken on Novartis Vaccine’s experimental MeNZB vaccine in New Zealand. Knowing this, Novartis Vaccines has clearly fabricated a headline for commercial and political reasons.
One effectiveness study in South Auckland was abruptly cancelled a year early on pretence that case numbers in both vaccinated and unvaccinated children had declined to the extent any results would be statistically insignificant.
The fact case numbers in unvaccinated children had also imploded should have suggested to MeNZB fundamentalists that the epidemic had waned naturally.
The South Auckland study was canned soon after principal investigator, Professor Diana Lennon, expressed distrust in Chiron Vaccines. Professor Lennon complained in writing to the Northern Ethics Committee, stating Chiron denied her access to her own clinical trial data, thereby preventing independent verification of Chiron analysis.
Furthermore, Professor Lennon complained that she was required to sign off research results without having seen the data. This is in breach of research code of ethics. Professor Lennon complained she first sighted the results of two of her four studies after Chiron and the Ministry of Health had used them to fast-track approval for the experimental MeNZB vaccine.
New Zealand parents should be very concerned when a senior researcher at New Zealand’s leading university, The University of Auckland, confesses in writing that such actions have occurred, are wrong and are in breach of the research code of ethics.
In essence, what has occurred is scientific misconduct involving New Zealand’s premiere University. An independent investigation of the circumstances surrounding this misconduct is needed urgently.
2. The “efficacy” figure of 80 percent also appears to be a very convenient figure. Using data available in the public domain, a nicely rounded figure of 80 percent is implausible.
The ‘desperate need’ for the experimental MeNZB vaccine was created by combining all cases in all age groups and all bacteria types, confirmed or not and packaging it up in a campaign of fear targeted at vulnerable children.
By contrast, Professor Lennon’s cancelled effectiveness study and a rushed Victoria University study were limited to confirmed cases of a single strain in a defined age group with a very early cut-off point.
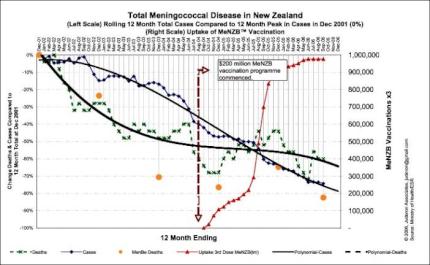
Most of the decline in case numbers occurred before the vaccine rollout.
Novartis Vaccines knows the MeNZB experiment has not impacted on total meningococcal disease in New Zealand.
Continuing to manipulate and falsify data serves to ramp up market presence and share price.
- Novartis Vaccines claims the MeNZB experiment has now concluded.
Four thousand healthy babies are still being injected with the experimental MeNZB toxin every week in New Zealand!
Nowhere in the entire history of medicine has there been a credible pharmaceutical experiment where analysis of over one million subjects was conceived in March, finished by June 30, with no control group, no randomization, no placebo and no double-blinding.
Despite this, Novartis Vaccines and the Ministry of Health would have us believe they have identified and verified and typed every case of meningococcal disease in under 20 year olds, and established the vaccination status of each case, including identifying ‘breakout’ cases (a vaccine fundamentalist euphemism for vaccine failure) reported from doctors, hospitals, and laboratories across the entire nation.
Novartis Vaccines and the Ministry of Health further expect us to believe that all data has been checked, analyzed, and peer reviewed by truly independent peer reviewers, produced conclusive results, and announced to the world; all without being published in a reputable peer reviewed medical journal and all within one month.
Not only is such a scenario implausible, it reeks of scientific fraud.
The rushed Victoria University effectiveness study was closed the week the main rollout finished… maybe sooner!
One month later New Zealand experienced the most deaths from meningococcal disease in a single month since 1997 and the highest number of cases in a month since the MeNZB experiment began. None of these later cases made it into the study data.
At the time of the study closure, fifteen MeNZB vaccine failures were reported. By the time results had been announced a month later that figure had all but doubled. It continues to rise.
Despite dumping the MeNZB vaccine, Novartis Vaccines still claims it as a success.
Novartis vaccines would have us believe that despite barely 70 percent of ‘high-risk’ Maori children having being vaccinated MeNZB was responsible for a miraculously high reduction of 90 percent.
The MeNZB vaccine does not confer herd immunity so this claim is clearly fabricated.
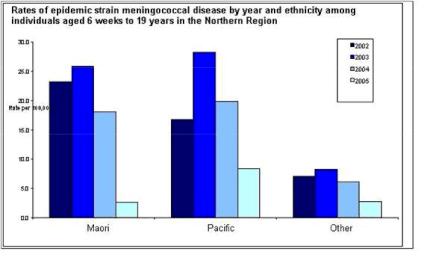
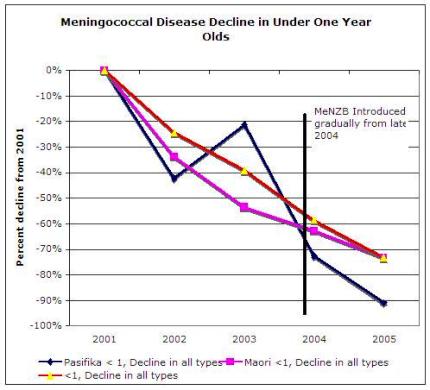
Miraculously, the Ministry of Health has used these exact figures to claim that cases declined by 90 and 70 percent respectively in these groups in the Northern region when comparing 2003 and 2005 yearly totals. [4]
These figures of 90 percent and 70 percent were derived before the Ministry of Health and Novartis Vaccines undertook their rushed effectiveness study.
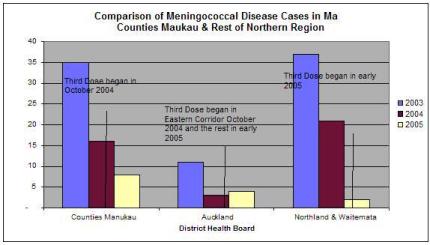
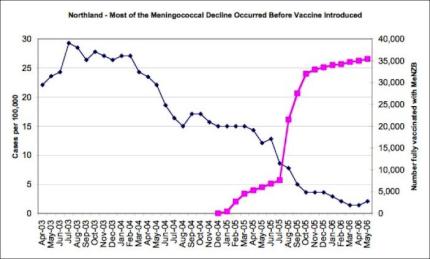
However, the majority of the decline, including confirmed cases of the so-called epidemic strain targeted by MeNZB, had occurred before the rollout of the experimental MeNZB vaccine with the decline in Counties Manukau District Health Board among Maori being comparable to that for Northland & Waitemata District Health Boards where no vaccine had been introduced. [5]
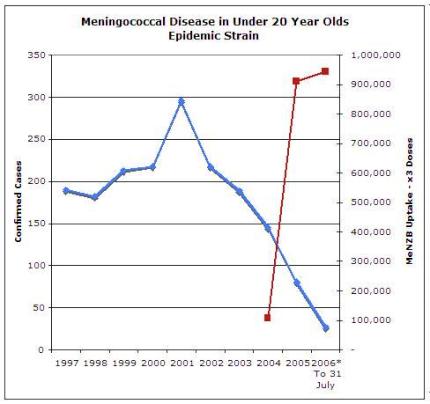
The freefall in case numbers continues unabated in the northern region, in all age groups and also in most regions, with Waikato being a notable exception. [6]
At the time of implementation of the MeNZB vaccine experiment, IMAC and the Ministry of Health claimed a simple tweak of the recipe would enable a similar tailor-made vaccine with any number of meningococcal B strains, “it’s just the same as the flu vaccine being changed each year” they said.
Why would a large global vaccine company abandon such a successful, profitable and easily contextualized vaccine?
Could it be that Chiron was offered a business opportunity that it simply couldn’t refuse? A home for three million doses of an orphan vaccine with a guarantee of total control over clinical trials, a State funded fear based marketing campaign, compliant officials and a fast-tracked license process?
The New Zealand government distorted the use of section 23 of the Medicine Act to enable a fast-track temporary license. This license is normally reserved for experimental or high-risk drugs for use in no-hope situations.
The experimental MeNZB vaccine has recently had its temporary license extended for another two years under Section 23.
If the evidence of the vaccine’s effectiveness and safety is so compelling why was it not given a full license under section 21 of the Medicines Act to enable all New Zealanders to benefit from this magic potion?
New Zealand was ripe for the picking. Chiron was able to join the meningococcal gold rush, expand its vaccine business and, in the process, make a cool $NZ140 million courtesy of the New Zealand taxpayer.
These figures are for all cases of meningococcal disease, confirmed and unconfirmed, all bacterial types and all age groups.
It is fraudulent to promote a strain specific vaccine targeting an age specific group in this way. Dr William Perea from the WHO has informed us that this is unscientific.
According to the Minister of Health [7] the death rate from the epidemic strain of meningococcal disease is 25-50 percent of other strains.
The death rate for the epidemic strain in New Zealand was approximately 1.5 percent before the introduction of the experimental MeNZB vaccine.
Since the introduction of the MeNZB vaccine the death rate has doubled raising the specter of mutation. This potential is noted in the recent UK media report quoting Novartis Vaccines researchers.
No New Zealand studies show such high disability rates. Data received under the Official Information Act and the medical literature shows such high morbidity relates to data from other strains and from other countries.
It is worth noting that images of the disease used to instill fear into school children were nearly all from overseas. The images depicted victims of meningococcal C, a strain of bacteria that causes significantly more morbidity than the strain targeted by the experimental MeNZB vaccine.
Using unrelated images so graphic that children of all ages went home begging parents to be allowed the vaccine is grossly unethical. It is in breach of the Nuremburg Code, to which New Zealand is a signatory.
It is worth noting no comprehensive study has been conducted on morbidity in relation to meningococcal patients in New Zealand. Available figures reveal nine amputations in children over a twelve-year period. The extent of those disabilities is not recorded though would have included individual digits.
Clinical follow-up showed that all children interviewed over 5 years of age attend ordinary regular school classes and were physically active within the context of their physical disabilities.
Other than the Chiron researcher at the ESR laboratories, we can find no evidence that the ‘epidemic’ strain of meningococcal B has ever resulted in an amputation. [8]
International studies show that rates of sequela are generally similar to fatality rate; the fatality rate for the epidemic strain of meningococcal disease in under 20 year olds in New Zealand is less than 2 percent. It is much less than 1 percent for 5-9 year olds.
Based on the available evidence, we can only assume that Novartis Vaccines continues to use fabricated and fraudulent claims of morbidity and mortality for commercial and political reasons.
Unresolved Issues:
Using a vaccine as a placebo is not good science and constitutes scientific fraud (although our research suggests that this unethical practice is common in vaccine trials).
Using an unlicensed vaccine as a placebo in newborn babies is highly unethical, if not illegal. It is certainly immoral.
The obvious conclusion: Chiron used this opportunity to undertake antibody experiments in New Zealand babies to expand their wider global vaccine business.
- Contrary to Novartis Vaccines and the MoH’s repeated protestations, there was no meningococcal disease epidemic in New Zealand as claimed.
The World Health Organisation has denied MoH claims that it [WHO] defines an epidemic as more than 3 cases per 100,000. Dr William Perea has stated in writing that no such WHO definition exists and that the Ministry of Health has created the definition.
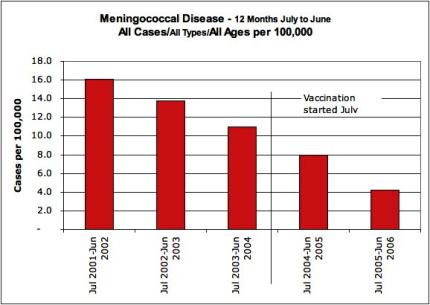
The natural decline in meningococcal disease in New Zealand was well in hand before the MeNZB rollout.
There was no social or medical reason for the $200 million MeNZB program. The need was a fabrication, generated though ‘fear marketing’ to create a market ripe for the picking. [9]
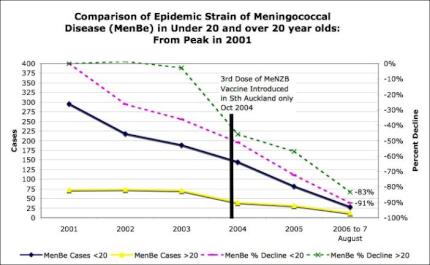
Cases due to the strain targeted by MeNZB in both under 20’s AND over 20’s were in significant free-fall decline before the experimental MeNZB vaccine roll-out.
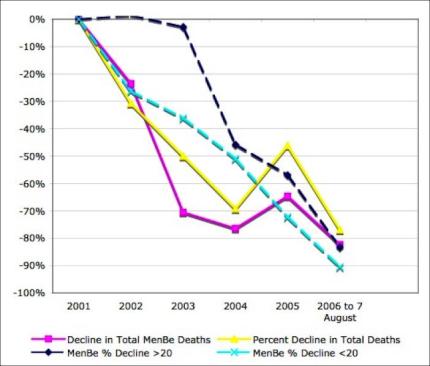
Cases had declined by 50 percent and deaths by 75 percent prior to the experimental MeNZB vaccine rollout. [10]
SUMMARY
Questions for Novartis
Vaccines:
1. Why did Chiron use their unlicensed meningococcal C vaccine as a "placebo" in healthy New Zealand babies during their trials in New Zealand?2. Did Novartis Vaccines undertake meningococcal C antibody testing of New Zealand babies given Chiron’s Menjugate C™ vaccine? If so, why?
3. Can Novartis Vaccines explain to concerned New Zealand parents why principal investigator Professor Lennon was forbidden, by Chiron, access to her own data in electronic form so that she could have it analysed independently of Chiron?
4. Why was Dr Lennon required to sign off research results without having seen the data?
5. Why, in some cases, did Professor Lennon first sight the results of studies she was responsible for, after those results had been presented to the licensing committee approving the MeNZB vaccine?
6. Can Novartis Vaccines explain to concerned parents of New Zealand why Novartis Vaccines and its supporters claim the natural decline in meningococcal disease is due to the effectiveness of MeNZB?
7. Does Novartis Vaccines understand the difference between the terms efficacy and effectiveness?
8. Will Novartis Vaccines confirm that no efficacy studies have ever been undertaken for its experimental MeNZB vaccine?
9. Can Novartis Vaccines explain why it publicly claimed 80 percent efficacy for its experimental MeNZB vaccine when no efficacy studies have ever been undertaken?
10. Does Novartis Vaccines agree that it is unscientific, unethical and fraudulent to use all cases of disease, confirmed or not, due to all bacterial types in all age groups to promote an experimental strain specific vaccine targeting an age specific group? If not, why not?
11. Can Novartis Vaccines explain why it continues to use such data when it knows that the death rate from the epidemic strain is, according to the Minister of Health [11] 25-50 percent of that due to other strains and the death rate for the epidemic strain in New Zealand is approximately 1.5 percent?
12. Can Novartis Vaccines explain to concerned parents of New Zealand where the company sourced statistics claiming that more than 1,000 New Zealanders have been permanently disabled by meningococcal disease?
13. Given all of the above, can Novartis Vaccines explain to the citizens of New Zealand why parents should continue to expose 4,000 healthy babies to this most unfortunate experiment every week?
The Meningococcal Gold Rush series I-III and MeNZB Quickguide can be viewed on-line:
- Meningococcal Gold Rush
I:
http://www.scoop.co.nz/stories/HL0502/S00064.htm - Meningococcal Gold Rush
II:
http://www.scoop.co.nz/stories/HL0607/S00284.htm - Meningococcal Gold Rush
III:
http://www.scoop.co.nz/stories/HL0505/S00352.htm - Meningococcal Gold Rush Quickguide:
http://www.sumnerburstyn.com/vax/MeNZB-Quick-Guide-332.pdf
FOOTNOTES:
1 http://www.scoop.co.nz/stories/HL0611/S00246.htm2 http://www.novartis-vaccines.com/press-room/news/20060808-MenZB.shtml or http://tinyurl.com/y8y6j5
3 http://www.scoop.co.nz/stories/GE0611/S00057.htm
4 http://www.immune.org.nz/?T=757
Click for big versionAbove graph is courtesy of the Ministry of Health and is on IMAC’s website and was distributed privately as early as May 2006, before any effectiveness study was undertaken. It is the Ministry of Health’s evidence of the effectiveness of MeNZB
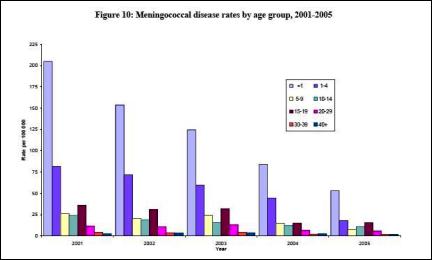
5
Click for big versionThe above data is from the Ministry of Health’s 2005 annual report on the epidemiology of Meningococcal Disease in New Zealand.
6 http://www.scoop.co.nz/stories/GE0611/S00006.htm
Click for big version7 Parliamentary Question 06029 (2005)
8 The strain of bacteria that infected Baby Charlotte, who was used by her parents and the Ministry of Health to promote the strain specific MeNZB vaccine, has never been publicly confirmed as being due to the epidemic strain. The TV3 Inside New Zealand documentary in 2005 noted that the strain had not been determined.
9
Click for big version10
Click for big version11 PQ 06029 (2005)


 Gordon Campbell: On Free Speech And Anti-Semitism
Gordon Campbell: On Free Speech And Anti-Semitism Ian Powell: The Disgrace Of The Hospice Care Funding Scandal
Ian Powell: The Disgrace Of The Hospice Care Funding Scandal Binoy Kampmark: Catching Israel Out - Gaza And The Madleen “Selfie” Protest
Binoy Kampmark: Catching Israel Out - Gaza And The Madleen “Selfie” Protest Ramzy Baroud: Gaza's 'Humanitarian' Façade - A Deceptive Ploy Unravels
Ramzy Baroud: Gaza's 'Humanitarian' Façade - A Deceptive Ploy Unravels Keith Rankin: Remembering New Zealand's Missing Tragedy
Keith Rankin: Remembering New Zealand's Missing Tragedy Gordon Campbell: On Why The Regulatory Standards Bill Should Be Dumped
Gordon Campbell: On Why The Regulatory Standards Bill Should Be Dumped