The Meningococcal Gold Rush - Second Edition
The Meningococcal Gold Rush, Second Edition
By Barbara Sumner Burstyn and Ron Law
Scoop Editors' Note: Following publication on Scoop.co.nz of Barbara Sumner Burstyn and Ron Law’s report, Investigation: The Meningococcal Gold Rush [1] - a report into the flawed science and bad policy of the MeNZB™ vaccine - the Ministry of Health declined to respond publicly.Instead the Ministry response was a private 14-page document sent to a number of medical practitioners and public health professionals.
Law & Sumner Burstyn published the MeNZB™ Quick Guide [2] via email and again the Ministry of Health declined to respond publicly. After increasing public and media publicity given to these reports, the Ministry of Health finally responded publicly to the MeNZB™ Quick Guide via www.scoop.co.nz on 24 May 2005. [3]
Following circulation of the authors’ two documents, a number of public meetings have been organized by ordinary concerned citizens at which one or other of the authors have spoken. [4] Total attendance has been in excess of one thousand concerned parents and health-care professionals.
Reprinted here in full is the Ministry of Health’s critique of The Meningococcal Gold Rush and Ron Law and Barbara Sumner Burstyn’s response. The authors refer to the MOH’s response to the MeNZB™ Quick Guide sparingly, mainly because to do so would have required a great deal of extra print without contributing significantly to the content.
Advertisement - scroll to continue readingThe editors of Scoop believe that important issues of public health should be debated in public.
The editors acknowledge that the following article is dense in content, but is well referenced. We recommend careful reading by those interested in issues of public accountability, informed consent, and safety and efficacy surrounding the MeNZB™ vaccine.
The editors particularly encourage readers to access documents with url links and contact the authors for verification of other references such as those obtained under the Official Information Act.
- Alastair Thompson For Scoop.co.nz
Summary
"The information parents and the public are being provided with regarding meningococcal disease and the MeNZB(tm) vaccine is seriously deficient. The Ministry of Health has developed policy and process with the transparency of a black box."
- Ron Law
Since publication of The Meningococcal Gold Rush the authors have continued in their investigations into the circumstances surrounding the contracting, development and implementation of the MeNZB™ vaccine.
The matters explored in detail in this new document, The Meningococcal Gold Rush, Second Edition, combined with new evidence of apparent undeclared conflicts of interest and our belief that the Ministry of Health has interfered with the independent functioning of the Health Research Council, form the basis of our call for a formal Royal Commission of Inquiry.
We believe the integrity, ethics, safety and justifications for the mass immunisation of 1.15 million New Zealand children is deeply flawed, dangerous, a violation of the principles of public health and informed consent, contrary to the Nuremburg Code, in breach of the Health and Disability Commissioner’s Code of Practice and various Acts of Parliament and therefore illegal.
It is our contention that the public has a vested interest in being fully informed on all issues surrounding the administration of an experimental medicine to all New Zealanders under the age of 20. This is required by international law.
The MeNZB™ vaccine was approved under a loop-hole in the Medicines Act that allows for restricted use of experimental drugs in a limited number of patients. This is anathema to good public health practice and is the very thing that international agreements signed by New Zealand, such as the Nuremburg Code, were designed to prevent.
The issues we raise in Meningococcal Gold Rush, Second Edition, warrant a full and prompt Royal Commission of Inquiry and we suggest a number of questions that such an inquiry should answer.
It is the authors’ contention that unless these issues are aired and resolved in a public and forthright way, the future integrity and functioning of the Ministry of Health and all vaccination programs will be greatly damaged.
The authors wish to publicly state, again, that they are not pursuing an anti-vaccination agenda, but rather drawing the public’s attention to what they believe is a gross abuse of administrative power.
The authors believe that the Ministry of Health is deliberately using Appeal Court Judgment CA232/96 - The Electoral Commission v L. A. Cameron and Others to mute even the guardian of published truth, the Advertising Standards Complaints Board when genuine concerns about false advertising by the Ministry are raised by the public. [5]
In the meantime, there appears to be sufficient evidence available to throw serious doubts on the integrity of the current MeNZB™ campaign from both clinical and process points of view.
The authors contend that the current mass vaccination program should be stopped forthwith and reconsidered in light of a full Royal Commission of Inquiry.
"The great tragedy of Science is the slaying of a beautiful hypothesis by an ugly fact."
(Thomas Huxley, 1825-1895)
The Meningococcal Gold Rush, Second Edition
In a private response to medical practitioners, the Ministry of Health has responded to the original report The Meningococcal Gold Rush. “MeNZB MOH response to gold rush long version 220205.doc” appears to have been written on or about 22 February 2005. File properties suggest the file had been circulated prior to 8 March 2005.
The following “ Fallacy” and “ Fact” sections (indented in this online version) are copied directly from the Ministry of Health’s document with The Meningococcal Gold Rush response following. Numbering is added for convenience.
In the document critiquing The Meningococcal Gold Rush and sent to GP’s and medical professionals:
1. The Ministry of Health says
Fallacy: Claims of obtaining secret documents and calls for a Royal Commission of Inquiry.Fact:The Office of Controller and Auditor-General has already rejected claims to investigate statements made by Ron Law that the Ministry of Health was manipulating epidemiology data. The Ministry responded in full to Ron Law, and copied these to the Office of Controller and Auditor General who were happy with the response
Meningococcal Gold Rush Authors
Respond:
There is no fallacy in secret documents.
Our call for a Royal Commission of Inquiry into the antics
that has foisted an experimental drug onto 1.15 million
otherwise healthy children - using a loophole in the
medicines act designed to permit such drugs for use on a
restricted basis and for a limited number of patients - is
certainly not a fallacy.
Information in The Meningococcal Gold Rush was sourced from documents primarily received under the Official Information Act. These documents would have remained unknown to the general public and the majority of the medical profession had they not been uncovered by the authors. [6]
A number of matters relating to the development and implementation of the MeNZB™ vaccine were referred to the Auditor General for comment.
The office of the Auditor General did not reject any claims. They did state that they were not arbiters of clinical issues and noted that it: “is unclear whether the MOH have had the opportunity to consider and respond to the allegations that have been made. We encourage you to put your case to the Ministry. We would then be happy to consider its response, and decide in the light of that reply whether or not to proceed with an inquiry into the matter ourselves.”
The Auditor General did NOT convey satisfaction regarding the MOH response as claimed by the Ministry. [7]
The MOH claimed on live radio that they did not have time to respond to the revelations. [8]
Despite this comment, as noted, the MOH responded directly to medical practitioners and public health professionals rather than engage in a public debate where their claims could be examined openly and verified or otherwise. It is noted that the MOH has belatedly responded publicly to the MeNZB™ Quick Guide. Responses to key issues are included in this report.
Claims of obtaining secret documents and calls for a Royal Commission of Inquiry are facts, not fallacies as claimed by the MOH.
- False declarations of competing interest made
by the authors of the cost benefit analysis (Cost Benefit
Analysis) used to convince Cabinet to fund the MeNZB™
vaccine and campaign.A formal complaint is being
prepared by The Meningococcal Gold Rush authors
regarding:
- False claims by MOH officials
that the Cost Benefit Analysis was independent. [9]
- Failure of key
advisors to declare their involvement in MeNZB™ research
being assessed during approval process.
2. The Ministry of Health says:
Fallacy: Claims that the total cost of the Meningococcal Vaccine Strategy had risen to $250 million.Fact: This is a misquote from an Opposition Party Member of Parliament. This figure has never been stated by the Ministry of Health or the Minister of Health Hon Annette King.
Meningococcal Gold Rush
Authors Respond:
The total costs statement was made
twice by the Associate Minister of Health (not an opposition
party member) in response to a parliamentary question. [10] Ministers’ responses
are normally prepared by government officials.
Note: MOH officials who wrote this response to The Meningococcal Gold Rush have not even bothered to verify the source provided with the [Gold Rush] document.
3. The Ministry of Health says:
Fallacy: Claims that the MeNZBTM vaccine was not developed by Chiron Corporation.Fact: Chiron has always worked in collaboration with Norwegian Institute of Public Health (NIPH) in the development of MeNZBTM, given the experience of NIPH in the development and testing of MenBVactm.
The Ministry of Health was actively in dialogue with the WHO in searching for a strain specific group B meningococcal vaccine from 1995. NIPH recognised early on that it did not have the ability to produce a NZ vaccine in the volumes required and so a partnership with Chiron Vaccines was formed.
Meningococcal Gold Rush Authors
Respond:
The MOH spin-doctors have tried to
obfuscate the issue. It is true that the NIPH did not have
capacity to mass-produce the volumes required for New
Zealand’s vaccination program. However, that was not the
issue. The issue is that false claims have been made that
Chiron developed the vaccine. They did not. The
simple irrefutable fact is that the NIPH developed the
vaccine in their labs in Norway and that Chiron are mass
producing it in Italy and marketing it. End of story.
To clarify the issue the MOH must declare when the NIPH/Chiron partnership was formed in relation to the MeNZB™ vaccine and exactly when and where the vaccine was developed.
It was clearly developed before the contract to [allegedly] develop it was approved by Cabinet [late 2001] and signed in early 2002. Our evidence indicates that the first batch of MeNZB™ vaccine used in the clinical trials was manufactured in Norway by the NIPH at least as early as September 2001, nearly two months before the Cabinet approved funding which included the development of the vaccine.
4. The Ministry of Health says:
Fallacy: Claims that Chiron will net around a cool $140 million for developing and supplying the already developed vaccine.Fact: This figure is complete guesswork. Chiron will get a significantly lower amount than guessed at in the article. However, the exact amount remains confidential as is commercially sensitive.
Meningococcal Gold Rush Authors
Respond:
This is not “complete guess-work” but does
involve reasoned assumptions. Not all financial details were
censored in one of the papers released under the Official
Information Act. The math is relatively simple. The figures
given to Cabinet was for $70 million to be paid for the
vaccine out of a total of $138 million. When the contract
with Chiron was announced the total cost had escalated to
$200 million. The difference is assumed to be due to
Chiron’s superior negotiating position and skills. These
figures imply that the Ministry of Health is paying
approximately $140 million for Chiron’s vaccine.
What’s more, the MOH committed to an exchange rate of US 40 cents. If Chiron was paid in NZ dollars then its return would be 80 percent higher than anticipated. If Chiron was paid in US dollars then the MOH should be crowing that the vaccine program was costing somewhat less than $200 million.
In June 2004 Dr O'Hallahan said that the amount Chiron was being paid for the MeNZB vaccine “was a significant proportion of the total budget.” [11]
5. The Ministry of Health says:
Fallacy: Claims that the MenBVactm vaccine produced by NIPH had insufficient efficacy to justify its use in a mass vaccination programme.Facts: While there was discussion on the efficacy results of the MenBVactm vaccine produced by NIPH – this was undertaken more than 15 years ago using a 2 dose schedule.
The results from that study showed an efficacy rate of 87 percent at 10 months. NIPH officials have stated that the decision not to introduce the Norwegian MenBVactm vaccine was made in the 19th year of their epidemic because there was evidence that the epidemic was waning naturally and a vaccine programme would not have been cost beneficial.
Since this time there has been several clinical trials using OMV vaccine technology that show that a 3-dose schedule improved the level of functional antibody response. The MeNZBtm clinical trials learnt from this and adopted a 3-dose primary vaccination schedule.
The results have been conclusive that in all age groups the high benchmark indicating level of antibody response has been exceeded.
Meningococcal Gold Rush
Authors Respond:
No efficacy studies have been
undertaken to show that a three-dose regime is more
effective than two doses. This is pure faith on the part of
the MOH.
In their cost benefit analysis the calculated benefit was based on 5 doses for under one year olds and four doses for every one else, and yet the costs were based on an average of just over 3 doses for under one year olds and less than 3 doses for over one year olds.
The Lancet medical journal simply stated that the efficacy wasn’t good enough for a mass immunisation program. [12]
If it was as simple as adding a booster shot to get satisfactory efficacy then why hasn’t the Norwegian vaccine been approved for use to this day… even though meningococcal deaths continue at higher rates than are occurring in New Zealand?
The natural decline in meningococcal cases in New Zealand is much greater than occurred in Norway by the time the vaccine was rejected, and deaths rates in Norway were more than double those in New Zealand. , [13] [14]
The MOH claims that trial results in New Zealand “have been conclusive that in all age groups the high benchmark indicating level of antibody response has been exceeded.”
The MOH should state publicly what its “high benchmark” was, and who determined the benchmark level. It is pseudo-science to claim in retrospect that a 55 percent antibody response rate, as occurred in under 6 month old children, is a “high benchmark.” Is the MOH statement, “the high benchmark indicating level of antibody response” another MOH statement of faith?
The MOH has never published what acceptable ‘benchmarks’ are. Setting arbitrary and undefined benchmarks retrospectively is not good science.
The juxtapositioning of the words ‘conclusively’ and ‘indicating’ is an oxymoron and is not the first time the MOH has demonstrated dichotic thinking. In May 2005, the MOH stated publicly, presumably to convince the public that the vaccine would work, that ‘efficacy was proven’, even though they know this to have been a false statement. [15]
At the same time, in their scientific publication, the MeNZB™ authors, who include the MOH spokesperson, state; “these findings suggest that MeNZB is safe and is likely to confer protection…” The use of the terms “suggest” and “likely” used in their scientific writings [16] is at odds with their use of the phrase, “Clinical trials have proven the vaccine is effective…” [17] for the public audience.
In its critique of the MeNZB™ Quick Guide the MOH stated, “The trials showed the vaccine to be safe and effective in generating a protective antibody response. This is reflected in the committee minutes when licensure was recommended.”
In reality the MAAC stated that there was no certainty that antibody levels detected in the trials would actually confer protection from meningococcal disease. [18] Contrary to what the MOH would have the public believe, MAAC never said that the antibody response was protective.
The MOH then went on to correctly say, “We will have estimates of effectiveness when the programme is complete…” [19]
In other words, they confess to not knowing whether the vaccine will work or not; put another way, they confirm that the MeNZB™ rollout is a medical experiment. While New Zealand parents have been told via MOH health advertisements that the MeNZB™ vaccine is “thoroughly tested.” This is surely fraudulent and needs to be addressed by the Royal Commission of Inquiry; especially as the MOH has hidden behind Appeal Court Judgment CA232/96 - The Electoral Commission v L. A. Cameron and Others to mute the guardian of published truth, the Advertising Standards Complaints Board when genuine concerns about false advertising by the Ministry are raised by the public. [20]
The Ministry has stated frequently that extensive studies of the MenBvac Norwegian vaccine have demonstrated efficacy. They have stated that the only reason the vaccine was not used in a mass campaign was because the epidemic was waning naturally.
A document received under the OIA shows this
was not the only reason. The minutes of the Vaccine Safety
Committee clearly state that international studies show:
- The efficacy in preventing invasive meningococcal
serotype B has not been proven.
- The effectiveness in
very young infants has not been proven.
- Very little
evidence of effectiveness.
- Its effectiveness has not
been proven,’
- Evidence of efficacy was not compelling.
[21]
Furthermore the MAAC document says that while serum bacterial antibody assays are the most reliable measure of functional antibodies following vaccination there is no international agreement as to what titre of antibody is protective. The use of antibodies to test clinical protection is so porous that it can even change depending on the particular batch of product. [22]
It is of interest that a MeNZB™ Principal Investigator and advisor to the Government and member of the licensing committee, [same person] stated in Chiron’s application for Clinical Trial V60P4 in mid 2003: “Furthermore, there is a huge clinical database for the parent vaccine, MenBvac, that indicates probable efficacy…” [our emphasis] Even Chiron wasn’t sure about efficacy. [23]
- One of the MOH’s key outside advisors, Professor Cartwright from the UK says that OMV meningococcal B vaccines are “ineffective in infants.” [24]
6. The Ministry of Health says:
Fallacy: Claims that there is a natural decline occurring in the disease incidence in New Zealand.Facts: There have been 228 deaths since the epidemic began
There have been more than 5600 cases of meningococcal disease since epidemic began
The epidemic strain accounts for about 75 percent of confirmed cases
For every 100 cases
Four die
20 are maimed and disfigured
76 recover with appropriate treatmentNew Zealand’s health professionals are also becoming proficient at diagnosing the disease meaning that many lives are able to be saved or disability avoided through early detection.
While cases of meningococcal disease have declined since a high year in 2001, the epidemic quite clearly continues. In 2004 the rate of disease was at more than three times the rate considered an epidemic by the World Health Organisation.
If this epidemic is not brought under control then New Zealand children will continue to die due to the epidemic strain of meningococcal disease and suffer the horrific consequences of this disease.
As observed in Norway, the pattern of group B meningococcal disease epidemics does not follow a normal epidemic curve but fluctuates with a number of peaks and troughs. New Zealand’s epidemic is no exception with the first peak of 613 cases and a rate of 16.9 cases per 100,000 population followed by a drop to 440 notified cases in 1998. 2001 recorded a second peak of 650 cases at 17.4/100,000. In 2002 the number of cases dropped by nearly 100 notified cases to 557 and in 2003 was 14.5 per 100,000 (541 cases). A further drop in numbers in 2004 does not mean that the epidemic has necessarily run its course. It is best practice to review data over extended periods to review changes in disease rates. It is always important to review the data in context of external conditions that may differ between years.
There are many factors that may contribute to an increase in the likelihood of someone contracting meningococcal disease. We know that across the board in 2004 the rates of respiratory diseases, which peak over the winter months, have been lower. Kidzfirst General Manager, Nettie Knetsch reported a 9 percent decrease in acute admission of young children over the past two winters when interviewed on Morning Report on Radio New Zealand, 18 November 2004. Rates of disease remain over 3 times epidemic levels.
Meningococcal Gold Rush Authors
Respond:
The evidence is irrefutable; there is a
natural decline occurring in the disease incidence in New
Zealand… End of story. , , [25] [26] [27]
It is bizarre that
the MOH would respond to a referenced article by saying that
this is a fallacy… especially when the information used was
their own provided under the OIA and through parliamentary
questions.
The Ministry then says;
There have been 228
deaths since the epidemic began
There have been more than
5600 cases of meningococcal disease since epidemic
began
The epidemic strain accounts for about 75 percent
of confirmed cases
For every 100 cases
Four die
20
are maimed and disfigured
76 recover with appropriate
treatment
Stating that there have been 228 deaths and more than 5600 since the epidemic began does not refute the fact that numbers of deaths and cases are in a free-fall decline.
The Ministry is, again, using all deaths from all strains to argue in favour of a type specific vaccine.
Since when do policy-makers use the fact that there have been 8,000 road traffic deaths since 1991 to justify policies? Imagine the outcry if the Ministry of Health correctly stated that since 1991 there have been an estimated 20,000 deaths in New Zealand due to highly preventable medical injury in our hospitals and incorrectly said that was evidence that our hospitals were getting safer?
The Ministry’s use of such figures is misleading, evidence of policy failure, and we would suggest warrants investigation [28] by a Royal Commission of Inquiry.
Barely 50 percent of all cases of meningococcal disease have been confirmed as being due to MenBe.
Seventy percent of confirmed cases equates to approximately 50 percent of all cases. The ESR itself argues that between 10 and 25 percent of all cases notified are false diagnoses. [29]
The death rate due to confirmed MenBe cases is approximately 1.5 percent for the past two years. We note with some satisfaction that the Minister of Health has recently confirmed our argument that, “It is well documented in epidemiological literature that the case-fatality rate for serogroup B meningococcal disease is between 2 and 4 times less than other meningococcal disease serogroups that have been reported in New Zealand.” [30]
Whilst we are surprised that the Minister has admitted the lower death rates this confirms statements we have made which officials have tried to rubbish by saying we weren’t qualified epidemiologists or acknowledged academics, or ‘real’ researchers.
The Minister’s answer provides compelling evidence that the continued use of a 4 percent death rate by officials to justify a strain specific vaccine with a much lower death rate is another example of misleading use of statistics warranting an inquiry. As discussed elsewhere, the MOH has recently ramped the death rate in children figure up to 5 percent.
Further, we note that the Ministry of Health continues to use historic data to argue a current solution to a diminishing public health problem. The death rate has been below 4 percent and falling since 1999. [31]
The Ministry of Health’s claims that the falling death rate is due to increased use of pre-admission antibiotics is also not congruent with the facts. [32]
It could easily be argued that a number of meningococcal deaths are the direct result of the fact that approximately 75 percent of patients do not receive the medical care that they should when presenting to a medical practitioner.
Unfortunately, in an answer, presumably written by Ministry of Health Officials, the Minister then makes the misleading statement; “However, the epidemic strain of group B meningococcal disease causes approximately 75 percent of notified cases in New Zealand.”
The Ministry of Health official(s) who supplied this answer would have known this to be factually incorrect; the epidemic strain of group B meningococcal disease causes approximately 75 percent of confirmed cases in New Zealand and confirmed cases of the epidemic strain of group B meningococcal disease account for as few as 50 percent of notified cases.
Using historic data to argue a current scenario is scientific and policy fraud.
We can find no evidence to support the claim that 20 percent of New Zealand cases are “maimed and disfigured.” Given the low fatality rates we doubt this is the case.
It can be inferred from the Minister’s answer to PQ 06029 (2005) that if death rates are 50 to 75 percent less for the epidemic strain than for other strains then so too are severe injuries such as “maimed and disfigured.”
We believe the use of overseas data relating to other strains of meningococcal bacteria to justify an experimental drug in New Zealand is scientific fraud and further evidence of misleading use of statistics. We challenge the MOH to produce their evidence to support this statement from New Zealand data.
This is urgent given the Ministry use of this statement along with video graphic colour photographs of worst-case Australian scenarios to scare parents and their children into silent acquiescence.
To say that 76 percent of cases recover with appropriate treatment is another hoax especially given that approximately 75 percent of cases do not receive appropriate treatment and many of the deaths are related to medical error in both diagnosis and treatment.
The MOH is using historic and global data to project onto New Zealand’s situation.
As acknowledged by the Minister, historically, meningococcal B disease has 50 to 75 percent lower death and injury rates than other strains. , [33] [34]
The MeNZB™ researchers have been aware of this fact for some time and yet the Ministry of Health falsifies the facts as they relate to a strain specific vaccine. It is likely that all adverse effects, major and minor, is less than or about 10 percent in New Zealand; somewhat less for the epidemic strain.
Evidence from the conveniently forgotten mid 1980’s epidemic [98/122 paediatric cases were meningococcal A] in Auckland revealed that despite a death rate of 7 percent, “If the acute illness was survived, the only detected long term sequela was sensorineural hearing loss seen in 6 percent.” [35]
Given the magnitude
of the stated problem in New Zealand, we find it
inconceivable that a competent health department would not
have undertaken studies to ascertain the facts as they
relate to New Zealand.
- Such an apparent omission
warrants the attention of the Royal Commission of
Inquiry.
In 2004 the rate of disease for MenBe was barely 1.5 times the rate considered an epidemic by the World Health Organisation, and was less than half the rate considered by the WHO as the threshold for introducing fully tested vaccinations; and bare in mind that MeNZB™ vaccine is an experimental drug.
Norway is not a typical example of an epidemic. The disease started in the north and slowly spread to the south resulting in a relatively flat curve. Any epidemiologist who has studied the epidemiology of the disease in Norway would have known this. In New Zealand cases rose more steeply and have fallen more quickly… as occurred recently in Ireland and in numerous other countries. Any epidemiologist who had done their homework would know this fact.
In saying “A further drop in numbers in 2004 does not mean that the epidemic has necessarily run its course” the MOH has at least opened its mind to the possibility that it has… especially with regards to MenBe.
In the past two years, most deaths have been caused by strains for which there are licensed vaccines in New Zealand. What political reason is behind the MOH’s refusal to advise the public of that fact?
The MOH claims that “It is best practice to review data over extended periods to review changes in disease rates.”
We agree… especially when the evidence is irrefutable that the epidemic was in steep decline… before the vaccine came into use. This can be seen in many DHB areas. Examples are produced here for reference. , , [36] [37] [38]
The Counties Manukau graph shows clearly that the MeNZB™ has had no noticeable impact on the spontaneous decline in total case numbers. The decline was substantial and well advanced before the vaccine was introduced and has continued perhaps more slowly.
The MOH then resorts to anecdotal evidence to try and make its case by quoting a radio report to justify an absurd statement. Whilst there might have been an unsubstantiated nine percent decrease in acute admission of young children over the past two winters, the fact is that during that time meningococcal disease cases have nearly halved and deaths have more than halved. The MOH should have actual data at its disposal, not rely on pseudo science such as unsubstantiated anecdotal media reports.
The authors find it bizarre that the MOH would provide the Meningitis Trust with the following so-called facts to use in their publicity material for promoting their upcoming appeal.
“Did you know?
Of 100 children who get Meningococcal disease:
1. 100 will spend from 2 - 50 days in hospital.
2. 5 will die.
3. 5 - 20 will develop severe brain damage or deafness. They may lose limbs or be left with damaged skin that needs extensive skin grafts.
4. 25 will be left with long-term learning or behavioural difficulties.
5. 60 will survive unharmed.” [39]- This data is scientific fabrication discussed further in point 23 and warrants the attention of a Royal Commission of Inquiry.
7. The Ministry of Health says:
Fallacy: Claims that the vaccine used in the roll out in New Zealand was not used in the clinical trials.Facts: The Chiron produced MeNZBtm vaccine was used in the New Zealand clinical trials and formed part of the licensure application. Certain clinical trials did involve the MeNZBtm vaccine produced at the NIPH site.
It is important to recognise that the MeNZBtm vaccine remains the same in production process and ingredients. On both sites a joint NIPH/Chiron team was involved in the development and production of the MeNZBtm vaccine. Each part of a clinical trial is designed to answer questions about a new product that are either unknown or require clarification.
The use of bridging information - or designing bridging trials, to obtain this information - is a standard process to help test a single hypothesis or set of hypotheses about a new product that is closely related to an original or “parent” product.
The design of the New Zealand MeNZBtm clinical trials was heavily influenced by existing information that was available on the parent MenBVactm vaccine, developed by the Norwegian Institute of Public Health, and other similar group B meningococcal vaccines using the same outer membrane vesicle (OMV) technology.
The Phase II MeNZBtm clinical studies bridged information between both NIPH MenBVactm and MeNZBtm vaccines and NIPH and CV produced MeNZBtm vaccines, which showed that MeNZBtm vaccines produced at both sites were comparable in results (safety and immunogenicity).
The clinical trials were carefully undertaken to the highest standard both ethically and clinically. There is a high quality post-surveillance monitoring system in place. The delivery of the vaccine is carefully controlled, not only through training of vaccinators and education staff, but also through the ongoing assessment by the ISMB independent to the Ministry of Health and DHB’s who are managing and delivering the MeNZBtm vaccine.
Meningococcal Gold Rush Authors
Respond:
The Ministry of Health’s spin-doctor is in
overdrive here.
According to the Minister’s Expert Advisory Committee that assessed the MeNZB™ vaccine, the Chiron produced MeNZBtm vaccine was not used in the New Zealand clinical trials that they assessed. The MAAC stated that only two small studies involved the Chiron produced MeNZBtm vaccine and that neither of those were completed when they assessed the vaccine. [40]
It concerns us greatly that the MOH is trying to mislead the medical industry into believing that the vaccine produced at the NIPH site played a minor role in the trials. According to the MAAC they were concerned that the Chiron produced vaccine wasn’t used in the main trials. It would appear that the MOH are deliberately obfuscating the facts.
It is of grave concern that documents released under the Official Information Act and only just received by us via other sources confirm this fact. These papers reveal that the Principal Investigator of one of the two [late] trials of the Chiron manufactured vaccine was none other than the Principal Advisor to the Ministry of Health with regard to the meningococcal Vaccine Programme. [41]
The same person is also a member of the MAAC Vaccine Subcommittee that approved licensure of the MeNZB™ vaccine, and despite not being a member of the full MAAC committee was included in the hurriedly arranged teleconference that rubber-stamped the recommendations of the MAAC vaccine subcommittee. This meeting was called with less than a day’s notice after the contentious vaccine subcommittee meeting. The meeting was so rushed that several members could not be contacted, and several others had not received the clinical data upon which assessment was based.
Neither the minutes of the subcommittee meeting, nor the minutes of the full committee meeting declare this previously unpublished conflict of interest; the minutes acknowledge his advisory role and then makes the bizarre statement that his participation had no bearing on the final outcome.
- We would expect a Royal Commission of
Inquiry to investigate whether false statements were made in
the application form regarding efficacy data when no such
data existed at the time. - We would expect a Royal
Commission of Inquiry to investigate whether false
statements were made in the application regarding an alleged
risk of ESR staff contracting meningococcal disease being
“estimated 500 times greater than person of equivalent age.”
(age range stated as being 18 to 50 years on page 7.) -
We would expect a Royal Commission of Inquiry to investigate
the appropriateness of a “sub-investigator” in the
Application for MeNZB™ trials working as a GP at the same
practice as the Principal Investigator. [42] - We would expect a
Royal Commission of Inquiry to investigate if it is
acceptable practice for a principal investigator of drug
experiments sponsored by a drug company to then be on the
independent regulatory approval committee for that
experimental drug. - We would expect a Royal Commission
of Inquiry to investigate if it is acceptable to not declare
that conflict of interest? - We would expect a Royal
Commission of Inquiry to investigate how much money was paid
to all investigators of MeNZB™ clinical trials or to
associated interests and whether those interests were
disclosed when required. - We would expect a Royal
Commission of Inquiry to investigate the appropriateness of
not disclosing those financial interests at MAAC meetings
and in other circumstances such as Ministry of Health
publications, Government briefings, conferences, [43] and medical journal
articles. (15) - We would expect a Royal Commission of
Inquiry to investigate whether the authors of the New
Zealand Medical Journal article submitted statements
declaring where potential conflicts of interest exist. And
if they stated explicitly all sources of funding as required
by the Journal. If so, why the New Zealand Medical Journal
failed to publish such information as they state is normal
practice? [44] - We
also believe that the Royal Commission of Inquiry needs to
investigate why the Ministry of Health permitted such a
conflict of interest to occur given that they would have
known this fact. Note: We have no evidence to suggest
that the Minister of Health was aware of these un-minuted
conflicts of interest.- We
would expect a Royal Commission of Inquiry to investigate
whether it is normal practice for a member of a subcommittee
of the licensing advisory committee that would approve any
license of a drug to not declare an involvement as a
principal investigator of that drug as noted in the
application.
The MOH says: “It is important to recognise that the MeNZBtm vaccine remains the same in production process and ingredients. On both sites a joint NIPH/Chiron team was involved in the development and production of the MeNZBtm vaccine.”
This is in marked contrast to the Minister’s statement in response to a parliamentary question that, “When a vaccine is produced at a new manufacturing site, it is deemed for clinical reasons a new vaccine and would require a full clinical investigation and licence application.” [45]
If the clinical evidence used to assess the MeNZB™ vaccine was so compelling, why did the Minister’s own expert committee express grave concern at the absence of efficacy data?
- We would expect a Royal Commission
of Inquiry to investigate this absence of efficacy data and
why, if the process to approve the MeNZB™ vaccine was so
robust, the minister refused to release the minutes of a
contentious MAAC vaccine subcommittee meeting the day before
the license was approved?
An informed source from with the Ministry of Health has stated off the record that the contention was due to undisclosed conflicts of interest.
8. The Ministry of Health says:
Fallacy: Claims there is something questionable about New Zealand not acquiring the intellectual property rights to the vaccine.Facts: NZ does not have the manufacturing ability to produce this vaccine and therefore has no benefit in owning the intellectual property for a vaccine that we cannot produce.
Meningococcal Gold Rush Authors
Respond:
This is a patently bizarre statement.
New Zealand has given away the genetic material used to develop and make the MeNZB™ vaccine. It would appear the MeNZB™ vaccine is intended to be redeveloped further for a global market. It is absurd to say that one doesn’t protect [acquire] intellectual property rights simply because one could not make it oneself.
Is the MOH ignorant of normal business practice? Was expert advice on intellectual property rights was sought when negotiating a $200 million dollar commercial project?
- We would
expect a Royal Commission of Inquiry to investigate if it is
standard practice for government entities to give many
millions of dollars of potential earnings from intellectual
property rights to corporate interests, and to determine the
full extent of this apparent
giveaway.
9. The Ministry of Health says:
Fallacy: Claims Chiron should be conducting new clinical trials on its own MeNZBtm vaccine in New Zealand.Facts:The Chiron produced MeNZBtm vaccine was used in the New Zealand clinical trials and the design of the clinical trials allowed the bridging of information between trials using the same vaccine but produced at different sites.
All clinical trials were run to Good Clinical Practice (GCP) compliance, the design was peer reviewed by international and national peer reviewers, and the MeNZBtm dossier was assessed by international experts before being seen by Medsafe.
The MeNZBtm vaccine being used in the roll out is made by Chiron and is the same as the vaccine used in the clinical trials.
Meningococcal Gold Rush Authors
Respond:
The Minister stated: “When a vaccine is
produced at a new manufacturing site, it is deemed for
clinical reasons a new vaccine and would require a full
clinical investigation and licence application.” [46]
Is the Ministry of Health saying that the Minister provides fallacious answers in response to parliamentary questions?
The MOH spin doctor’s claim that “The MeNZBtm vaccine being used in the roll out is made by Chiron and is the same as the vaccine used in the clinical trials” does not align with official documents released by the Minister under the Official Information Act.
According to the minutes of the vaccine subcommittee of the MAAC the Chiron manufactured vaccine was not used in the bulk of the trials and the two that had included the vaccine had not been completed when they assessed the data; one of those studies involved only 10 adults. New documents released under the Official Information Act confirm that. [47]
10. The Ministry of Health says:
Fallacy: Claims about the number of people in the clinical trials who received MeNZBtmFacts: Approximately 80% of the nearly 1700 people involved in the clinical trials received the MeNZBtm vaccine. The vaccine was produced both in Norway by NIPH and in Italy by Chiron Vaccines.
Meningococcal Gold Rush Authors
Respond:
It is an indictment on the Ministry of
Health when they can only come up with “approximately,” and
“nearly” in response to a so-called fallacy about how many
people actually received what.
We challenge the Ministry
of Health to state publicly EXACTLY:
1. How many of each
age group received the NIPH MeNZB vaccine?
2. How many of
each age group received the Chiron manufactured MeNZB
vaccine?
3. How many of each age group received each of
the other control vaccines including Chiron’s unlicensed
Menjugate MenC vaccine?
4. How many of each age group
received a placebo (as is the gold standard in medicine
research)?
5. How many of each age group were included in
double blind studies (as is the gold standard in medicine
research)?
We challenge the MOH to state publicly how many studies had been completed using Chiron manufactured MeNZB™ vaccine at the time that the vaccine was assessed by the MAAC vaccine subcommittee.
We challenge the MOH to release the Minutes of the secret MAAC subcommittee meeting held on 5 July 2004.
As noted, this meeting was so contentious that the Minister refused to release the minutes so that experts could have robust debate. The letter stated, “…the withholding of the information is necessary to maintain the effective conduct of public affairs through the free and frank expression of opinions …”
- A Royal Commission of
Inquiry should answer the question, “why was the full
committee meeting called with such haste that some members
could not be contacted and members, other than those on the
subcommittee, had not received the documents that were used
as the basis of approval?” - If the case in favour of
licensing the MeNZB™ vaccine was so robust, why was it
necessary for the government’s principal MeNZB™ advisor and
Chiron’s MeNZB™ principal investigator – to be present at
the full MAAC committee meeting when he is not a full member
of that committee? - And why was the MeNZB™ vaccine
licensed on the basis of a verbal report of the
teleconference meeting, and not a written
report?- A
Royal Commission of Inquiry should answer the question, “why
was that meeting not mentioned in the minutes of the full
teleconference committee meeting called after the secret
meeting and held the next day?”
11. The Ministry of Health says:
Fallacy: Claims about the vaccine efficacy and that the decision to license and the roll out were taken on insufficient data.Facts: Statements in the article and quotes selectively lifted from minutes don’t relate to the final dossier submitted as the results from the clinical trial were submitted in a rolling manner.
Experience with various meningococcal vaccines, A and C polysaccharide, outer membrane protein B vaccines in Brazil and Chile, suggests that the production of serum bactericidal antibodies correlates well with protection though probably underestimates the actual level of protection.
Furthermore, the safety data available on the Cuban vaccine in Cuba distributed in 65 million doses in Latin America, and the Norwegian vaccine in Norway, Iceland and Chile and the RIVM vaccine in the Netherlands and the UK is of relevance to the possible licensure of New Zealand strain vaccine.
International peer review was strongly in support of the New Zealand approach given the high rate of meningococcal disease dominated by a single strain of serogroup B meningococcal disease.
The approach to licensing a vaccine following immunogenicity trials was used by the UK authorities prior to the introduction of the Meningococcal C vaccine programme in the UK. This approach was similar to the New Zealand strategy when introducing the MeNZBTM vaccine.
Meningococcal Gold Rush Authors
Respond:
Using the Ministry of Health’s logic Vioxx
is Celebrex is Bextra is…was safe until the whistle was
blown.
We know that serious adverse effects and vaccine failures are stripped from official data. For example, we have been reliably told by Auckland Hospital staff that a 16 year old boy who was fully vaccinated with the MeNZB™ vaccine was admitted to the emergency department with classical meningococcal disease symptoms. We are told that he was treated for MD, and that he recovered and that the diagnosis in his charts was not meningococcal disease and that staff were told to keep quiet.
We are reliably informed that a fully vaccinated baby has recently died of ‘meningococcal-like’ disease in the Auckland region [not the 5 year old “protected” child who died of MenC] but that staff have been instructed to remain quiet.
We are aware of a 20 month old vaccinated infant who recently died from meningococcal disease in Starship.
We know that the 5 year old who died of MenC was not given antibiotics by either the ambulance or the ED because she had been vaccinated and that “it wouldn’t be meningococcal disease.” We also know that the child’s family was asked by medical staff and officials to keep quiet and not to go to the media when they realized that medical error had a role to play in the death.
Was there a coronial inquiry in this case? If not, why not? Given that S4(1)(c) of the Coroners Act 1988 requires every death—(i) That occurred while the person concerned was undergoing a medical, surgical, or dental operation or procedure or some similar operation or procedure; or (ii) That appears to have been a result of any such operation or procedure; or…”
If the above claim about failure to provide reasonable care such as antibiotics is true then this death should have been referred to a Coroner.
- We would expect a Royal
Commission of Inquiry to investigate whether this is an
isolated incident and if not if there are further deaths
that should have been referred to a
coroner.
We know that even injecting water into 65 million children as a placebo would cause serious adverse reactions so how credible is it for the Ministry of Health to use Cuban data to justify an essentially untested experimental drug in a first world country such as New Zealand? Claims that 65 million doses of any injectable medicine have not resulted in serious adverse events are implausible and could only result from selective filtering of adverse reports to sanitise any analysis.
- The fact that the Ministry
of Health has bypassed its own adverse event causality
assessment protocol also warrants the attention of the Royal
Commission of Inquiry.
The public has been led to believe that the Independent Safety Monitoring Board is reviewing all adverse reactions following vaccination.
We have received evidence that the Ministry of Health, Chiron and the MeNZB™ researchers and advisors have further interfered with due process and appointed a hand selected group to filter all reaction reports before they even get to the hand chosen ISMB. [48] These reports then go through the Ministry of Health’s Data Management Group (DMG) in the Meningococcal Vaccine Strategy team [Managed by Dr Jane O’Hallahan] and manages all flows of data in relation to vaccine effectiveness and safety including that which goes to the ISMB. , [49] [50]
- This also warrants the attention of
the Royal Commission of Inquiry.
- Why has there been a
systematic betrayal of public confidence in the independent
safety monitoring of the MeNZB™ vaccine?
12. The Ministry of Health says:
Fallacy: Claims made by quoting minutes of Medsafe subcommittee’s out of context such as: "there are a number of issues relating to the manufacturing and quality data that are to be addressed by Chiron".Facts:The assessment process takes months of consideration engaging international experts and an ongoing discussion with the vaccine manufacturer on the results – or further information. This is standard process. It did not take one day as alluded by this article.
Meningococcal Gold Rush Authors
Respond:
The MAAC is not a subcommittee of Medsafe.
It is a statutory committee established under the Medicines
Act to advise the Minister.
The Meningococcal Gold Rush Authors have not taken the minutes out of context at all. The MAAC met the day before the vaccine was licensed and noted these concerns in its minutes.
If the MAAC’s concerns had been addressed prior to the meeting that should not have been an issue noted in the minutes.
MeNZB™ was licensed the day after the meeting in which concerns were raised.
The safety concerns had not been addressed by the time the vaccine was licensed… and the first 300,000 doses would appear to have been manufactured before any safety issues could have been addressed.
Given Chiron’s woes in the UK and Italian manufacturing plants at the same time, as manifest by massive recalls involving more than 50 million doses in the UK and Brazil, our findings are reasonable.
13. The Ministry of Health says:
Fallacy: Claims questioning the vaccine safety.Facts: Results have validated that this vaccine is safe with more than 475,000 doses delivered up to February 6, 2005. To date in the Programme there have been no safety concerns.
Meningococcal Gold Rush Authors
Respond:
What confidence can there be in a system
that replaces the Health Research Council’s standing safety
monitoring committee with hand picked colleagues and
associates of key MeNZB™ researchers and advisors?
What confidence can the public have when the Ministry of Health, Chiron, MeNZB™ researchers and advisors then appoint, with no public notification, a hand chosen group to filter all adverse reactions reports before they go to the Health Research Council’s ‘Independent’ committee.
It would appear as if the HRC is simply a conduit for MOH money to create a façade of transparency.
Questions the Royal Commission of Inquiry needs to address
include;
- Why did the HRC not use its core safety drug
monitoring committee to assess the safety of MeNZB™?
-
Why did the Ministry of Health, Chiron and MeNZB™
researchers and advisors create an intermediary between
adverse reaction reports and the ISMB?
- Was the HRC
used by the MOH to create an air of independence in the
monitoring of the MeNZB™ vaccine?
- What confidence can
there be in a pharmaco-vigilance process that contains no
acknowledged experts in
pharmaco-vigilance?
14. The Ministry of Health says:
Fallacy: Claims questioning the expiry date of the vaccine.Facts: The claims show a lack of understanding on how shelf life is calculated and assessed. The stability of the vaccine is assessed through a rolling process. Prior to the licensure the latest data available was only up to 12 months to allow a 12-month shelf life, although the 18-month data came through a few days before the 8 July 2004 licensure announcement and therefore the shelf life was also able to be extended to 18 months. This is standard practice.
Meningococcal Gold Rush
Authors Respond:
The MOH should release documents
to support its claims.
This is not standard practice. The shelf life was extended solely to prevent the destruction of approximately 300,000 doses of vaccine that would have impacted on the rollout of the vaccine. According to a New Zealand Herald report, the vaccine shelf life was extended by 18 months, not to 18 months. [51] Regardless, the only reason for extending it was to save face and dollars.
15. The Ministry of Health says:
Fallacy: Claims about Chiron and regulators in the United States, Britain and Brazil.Facts: The article alludes to connections that do not exist.
Chiron did cease production of doses of flu vaccine destined for the US market because they became contaminated in production and the Brazilian government has removed the Chiron manufactured MMR vaccine from a mass vaccination programme.
Brazilian health officials stopped the use of Chiron's triple vaccine against measles, mumps and rubella, after an unexpectedly high number of children who received it experienced serious allergic reactions in an immunisation programme 2 weeks ago. There were no deaths reported. Chiron and Brazilian health officials are investigating the cases of at least 125 children who experienced the reactions.
The rates of adverse reactions were significantly higher among the children receiving the Chiron vaccine, which is made in Italy, than among children who received a vaccine made by another company. This is first episode of any problems reported from Chiron's MMR vaccine. The approach taken by Chiron is appropriate.
Chiron has not only acknowledged the issue, but has responded appropriately and a full investigation is underway.
The influenza vaccine was manufactured in a UK facility that Chiron has just bought from a previous manufacture. This factory has no links with the NZ MeNZBtm production in Siena, Italy. The Siena site has undergone several audits and complies with the Good Manufacturing Practice guidelines. All batches of the MeNZBtm vaccine that leave New Zealand are physico-chemically characterised to ensure the vaccine meets the high quality standards required by Medsafe, the New Zealand Medicines and Medical Devices Safety Authority.
Chiron has responded appropriately in investigating the issues and put on hold supply of both the MMR vaccine in Brazil and Influenza vaccine in the United States.
Meningococcal Gold Rush Authors
Response:
Our claims are substantiated and public
knowledge. The allusion of connections is very real; all
involved substandard manufacturing practices at Chiron
factories.
Chiron ceased production of its flu vaccine, not because it responded responsibly to contaminated flu vaccine, but because it didn’t.
UK regulators were forced to revoke its Good Manufacturing Practice certificate and closed the factory down.
The MOH statement of evidence regarding the Brazilian recall of Chiron’s MMR vaccine appears to be a media report at the time and is markedly similar to a New York Times article. [52]
It does not instill public confidence when the MOH appears to plagiarise media reports referring to events that happened nine months ago as, “experienced serious allergic reactions in an immunisation programme 2 weeks ago.” [MGR emphasis]
Has the MOH not even bothered to get an official response from Chiron?
This is especially significant given that the MeNZB™ experimental vaccine is made in the same Italian factory.
16. The Ministry of Health says:
Fallacy: Claims about the Ministry's cost benefit analysis being based on five doses.Facts: The Meningococcal B Immunisation Programme involves people receiving three doses of the vaccine. Work is continuing to decide if a fourth booster dose will be required for younger infants.
The cost-utility analysis included a large range of variables in the economic analysis measuring over 500 fields. While the report did include a primary series and a two dose booster series - the report only modeled information that was available at the time. Since then clarity on a range input assumptions would see a change in a range of variables if remodeling would occur. The Cabinet paper states on many occasions “if it were necessary, patients would receive boosters to give them continued protection.”
Meningococcal Gold Rush Authors
Response:
The ‘Fact’ presented here by the MOH has
no connection with the so-called ‘fallacy’ stated.
The fact that the benefit side of the Ministry of Health’s cost benefit analysis was based on five doses for under ones and four doses for every one else is a matter of record.
The fact that costs were based on fewer doses on average is also a matter of record.
The fact that Cabinet was told in the appendix of the Cabinet paper, that three doses were necessary, but that, “It is likely that all under ones will require a booster dose one year later…It is unlikely that a booster in the 1-5 year olds will be required” is also a matter of record
We can find no evidence in the Cabinet paper where ‘it states on many occasions 'if it were necessary, patients would receive boosters to give them continued protection.'’
Unless there are Cabinet papers that have not been released, the MOH response is dishonest. The cost benefit analysis was based on a five/four dose regime; Cabinet was told that only three doses were planned with a possible booster for under ones.
Informed sources at Auckland University tell us that a fourth dose has already been decided on for under six month old babies. This was alluded to by Dr Jane O’Hallahan at a public meeting in the Hawkes Bay on April 12.
Further more, the cost benefit analysis used numerous assumptions given to the authors by the Ministry of Health. Despite a 5/4 dose regime used in their modeling, they assumed that efficacy would disappear by year five. The Ministry of Health sold the vaccine to Cabinet on the basis that efficacy would last between five and ten years. [53]
The MOH says that the report only modeled information that was available at the time. The report itself states that it was based on assumptions provided by the MOH. Nearly all assumptions have proven false.
For example, the authors of a paper on the MeNZB™ vaccine in the August 2004 edition of the New Zealand Medical Journal stated, “Assuming vaccine efficacy of 80 percent and coverage of 90 percent of the eligible population with three doses of MeNZB™, the Programme aims to reduce cases of B:4:P1.7b,4 meningococcal disease in those aged less than 20 years by over 70 percent. This strain accounted for an estimated 72 percent of all meningococcal disease cases in 2003.” [54]
Based on these assumptions simple math, would reveal that the vaccine campaign is only expected to prevent .7 x .72 = 50.4 percent of cases in meningococcal disease in under twenty year olds which accounted for 73.4 percent of all cases in 2003. [55] This being the case, then the expectation of the researchers and the Ministry of Health is that the MeNZB™ vaccine will reduce the incidence of meningococcal disease in New Zealand by only 37 percent. (0.504x.734)
And yet the public has been sold the vaccine on the false hope that it would “stop the epidemic.” [56]
- This appears to be a major breach
of public confidence, and public health fraud that needs to
be investigated by the Royal Commission of
Inquiry.
Based on the 342 cases in 2004 that would mean that the vaccine is expected by officials to reduce the number of cases by 127 cases to 342x.37 = 215 cases still some 3-4 times above the pre-epidemic levels that are mentioned in ad infinitum by officials.
The Ministry of Health recently published in The Press that the MeNZB™ vaccine was expected to prevent 3,500 cases, 190 deaths and 600 under 20 year olds being permanently disabled. [57]
Let's do some more simple maths.
In 2003 and 2004 there were 9 deaths confirmed as being due to the epidemic strain of bacteria targeted by the MeNZB(tm) vaccine (5 & 4 respectively). Maybe 50 percent of these were in under 20 year olds making a total of 2-3 per year. Over 10 years that is 20-30 deaths that could, theoretically, be prevented if the vaccine works.
To get a figure of 190 deaths the Ministry of Health appears to have resorted to plucking figures from somewhere and projecting that over more than 10 years. That is pure science fiction. The maximum confirmed number of deaths - in all age groups due to the epidemic strain of meningococcal disease - was in 2001 when 17 people died.
Based on the Minister’s answer to PQ 06029 (2005), none of the five meningococcal deaths so far this year could have been prevented by the current MeNZB™ vaccine program. The two deaths in under 20 year olds were due to MenC and the deaths due to the epidemic strain were in over twenty year olds.
Dr O'Hallahan's 190 deaths divided by 3,500 cases gives a case fatality rate of 5.4 percent. As already noted, the death rate for all types of meningococcal disease for the past three years has been less than half that figure and is falling. The deaths rate for the epidemic strain is approximately 1.5 percent. As noted also in answer to parliamentary question 06029 (2005), the death rate from the epidemic strain of meningococcal disease is 25-50 percent of that due to other strains.
It would appear that the MeNZB™ vaccine will not reduce deaths by more than 1 or 2 per year based on 2003, 2004 and 2005 ytd data. Over ten years that equates to a maximum of 10-20 deaths assuming the significant natural decline in cases and deaths does not continue. This suggests that the Ministry of health is exaggerating the benefits of the MeNZB™ vaccine up to 1,800 percent.
During that same period some 500 babies will die of Cot Death and some 7,000 children will die from all causes.
The Ministry of Health also states in The Press, that "the vaccine has proved to be effective in clinical trials..." This statement is made knowing that it is totally false and that the trials did not establish either efficacy or effectiveness.
- We would expect a
Royal Commission of Inquiry to investigate why the Ministry
of Health is amplifying the risks associated with the
epidemic strain of meningococcal disease, along with
exaggerating the benefits by more than a thousand percent
and making fictitious claims to the public in an effort to
sell an experimental
drug.
17. The Ministry of Health says:
Fallacy: Claims about various conflicts of interest.Facts: The Ministry has always taken every necessary step to ensure there is no conflict of interest for people dealing with decision making around the vaccine a Programme design. However, there is a limited number of experts in meningococcal disease and immunisation so there were times when someone would have played a role in some part of the strategy but for conflict of interest reasons a separation of advice and decision making has been carefully followed.
Meningococcal Gold Rush Authors
Respond:
Staff at the faculty that stood to gain
upwards of five - ten million dollars in long-term funding
declared in writing in the Cost Benefit Analysis report;
“Competing interests: None.” [58]
This Cost Benefit Analysis was used by the MOH to discredit Treasury objections to the MeNZB™ vaccine programme, and to convince Cabinet to spend $200 million taxpayer’s money.
The lead author was not a MeNZB™ researcher but works in the same faculty and appears to have provided services as a private contractor. The Principal MeNZB™ researcher was a co-author.
When researchers attract large funding to a faculty, promotion and international status becon. The IMAC website has the program for the September 2001 immunisation conference. It contains the following;
“Attacking New Zealand's most urgent infectious disease issue: is a solution in sight? Professor Diana Lennon, Dr Jane O'Hallahan on behalf of the Meningococcal Management Team: Diana Lennon, Principal Investigator; Jane O'Hallahan, Ministry of Health; Philipp Oster, Chiron Vaccines; [with advisors Sue Crengle, NHC; Diana Martin, ESR, Teuila Percival, South Auckland Health; Stewart Reid, General Practitioner; Joanna Stewart, University of Auckland] [59]
It is important to realise that this is 3 months before cabinet gave their approval for the $200 million meningococcal vaccination project. The Meningococcal Management Team was already established, before Cabinet gave approval for the funding of meningococcal vaccination project.
- We would also expect a
Royal Commission of Inquiry to investigate if it is
acceptable for MOH staff to tell Cabinet that the Cost
Benefit Analysis was independent without drawing potential
conflicts of interest to the attention of Cabinet,
especially when the expenditure such large sums of money are
involved. - We would expect a Royal Commission of Inquiry
to answer the question, ‘why was the economic impact
assessment not put out to competitive
tender?'- The fact that the authors
declared that there were no competing interests knowing that
a member of the Meningococcal Management Team, which
included Chiron, was a co-author of such a pivotal document
is puzzling to say the least and warrants the attention of a
Royal Commission of Inquiry.
When threats are made to Universities that result in independent academic analyses being withdrawn from the public domain to protect the career interests of graduates, then serious questions need to be asked about competing interests; this should also be subject to a formal Royal Commission of Inquiry.
The MOH claims that “… for conflict of interest reasons a separation of advice and decision making has been carefully followed” is at odds with the facts.
When papers released under the Official Information Act reveal that key advisers are also the drug company’s principal investigators and are also on licensing committees - then we would argue that the ‘separation’ is a myth and the MOH has failed the public of New Zealand.
- We would expect a Royal
Commission of Inquiry to investigate all conflicts of
interest including those detailed in section seven of this
report.
18. The Ministry of Heath says:
Fallacy: Claims that another cost benefit analysis by Treasury in 2001 showed that the cost-to-benefit ratios were seven times those normally used by Pharmac to approve funding of prescription medicinesFacts: Pharmac does not have a set funding benchmark. The figure of seven times – is misrepresenting the true figure of ongoing immunisation to the highest risk age population – when design of Programme was matched with modeling assumptions.
Meningococcal Gold Rush Authors
Respond:
The claim that another cost benefit
analysis by Treasury in 2001 showed that the cost-to-benefit
ratios were seven times those normally used by Pharmac to
approve funding of prescription medicines is not a fallacy.
It is on record in Cabinet memoranda that Treasury believed that the government would get a much better social dividend for tax payers money and Treasury documents received under the Official Information Act allow calculation of the “seven times” claims. [60]
The modeling assumption has proven to be grossly false. For example;
1.
It was [falsely] assumed that more deaths would be prevented
than actually occurred or have occurred due to the MenBe
strain.
2. It was [falsely] assumed that the benefits
would save approximately 10 times the number of deaths that
could have been prevented during the past two years.
3.
It was [falsely] assumed that disease levels would remain at
peak [2001] levels for ten years.
4. It was [falsely]
stated that the epidemic was caused by a single strain of
bacteria.
If the Cost Benefit Analysis had utilized data from the past two years then the costs would be about 50 times Pharmac’s normal benchmark.
- The Royal Commission of
Inquiry should determine whether the MOH Cost Benefit
Analysis was based almost exclusively on false assumptions
and whether Treasury’s advice was
sound.
19. The Ministry of Health says:
Fallacies: Claims that a university student’s cost benefit paper was withdraw from the public and that the University received a threatening letter 'advising against publication.'Facts: An Honours student at Canterbury did a paper on the meningococcal vaccine decision. However, the paper was not a cost benefit analysis. Rather it carried out a review of the earlier modelling work done for the Ministry, including of assumptions made and conclusions, and of processes and decision-making. The paper was originally produced just as an internal piece of work for the course. However, the paper was presented at a conference and effectively entered the public domain.
Consequently the flaws and shortcomings then became an issue. When a paper is published or presented publicly, the author needs to be careful to check facts and interpretations, and it is good practice to share drafts for peer review, including with the authors and those involved in the original research. The author in this case did not follow good practice. The paper was presented, and raised some interesting issues. Unfortunately it contained errors of fact and interpretation, it confused the roles of the Ministry staff, the researchers, and the Ministers. It also contained potentially defamatory statements.
Subsequently the abstract was withdrawn from the website, and the paper was not circulated any further.
Meningococcal Gold Rush Authors
Respond:
Is the Ministry of Health claiming that
the Honours student’s paper was fallacious?
We put this response to the Canterbury University lecturer who supervised the student’s work who responded; “in my judgement there is nothing defamatory in anything [the student] has written. I like, and stand fully behind, what you wrote." [in our draft response sent to Canterbury university for verification].
Canterbury University has assured us that the reason the paper was withdrawn was to protect the career interests of the student concerned, not because the study was flawed. [61]
It is our contention that the MOH’s cost benefit analysis was based solely on hypothesis and wild assumptions that not only could not stack up, but that have been shown to be unsound.
We contend that it is the MOH’s cost benefit analysis that contained no substantive fact and is based exclusively on unsubstantiated assumptions and hypothetical interpretation.
We note also that the Ministry of Health states, “When a paper is published or presented publicly, the author needs to be careful to check facts and interpretations, and it is good practice to share drafts for peer review.”
We would suggest that it is hypocritical of the Ministry of Health to ignore its own advice by not checking its facts before surreptitiously replying to The Meningococcal Gold Rush whilst telling the media and the public that it didn’t have the time and resources to respond.
We note that the Canterbury University Student’s paper was presented to the NZ Association of Economists (Inc) conference in Wellington in June 2004 and entered for the Jan Whitwell prize. It was so well received that it was presented a second time by invitation at an event organised by Victoria University.
We suggest that having made a potentially libellous statement about the Hons. student’s paper and presentation, the Ministry of Health should release the paper in full with all Ministry of Health papers, communications with the MOH’s Cost Benefit Analysis authors, emails and correspondence relating to the paper: “Eradicating meningococcal disease in New Zealand: Is it worth it? To whom? And who decides?” presented at the NZ Association of Economists (Inc) conference in Wellington in June 2004.
- Under the Official
Information Act, we formally ask that the Ministry of Health
release all such papers to
us.
20. The Ministry of Health says:
Fallacy: Claims that the student was approached by officials from other government departments and congratulated for raising questions they were not allowed to.Facts: There are no questions that officials or researchers have not been allowed to ask. The research was available to Treasury and other officials at the time of the Cabinet decision on the meningococcal vaccine. Treasury and the Ministry of Health discussed drafts of Ministerial briefings, and Treasury provided their advice to Cabinet in the usual way.
Meningococcal Gold Rush Authors Respond:
We are reliably informed that the student was praised by officials from Pharmac for raising questions that they were not allowed to. We understand that Treasury officials also made complementary comments on the paper.
The student’s paper was researched as part of an economics Honours degree in 2003 and presented at a conference in Wellington in June 2004; the Cabinet decision was made in 2001.
21. The Ministry of Health says:
Fallacies: Claims that the Ministry used incorrect figures on the total number of cases.Facts: Not all cases were caused by the epidemic strain, but of the 5658 cases to date in the epidemic where a isolate could be taken – approximately 75 percent relate to the single B:4:P1.7b,4 strain of meningococcal disease.
It is very clear from the evidence that New Zealand has experienced a monoclonal epidemic caused by the B:4:P1.7b,4 bacterium. The Institute of Environmental Science and Research Limited (ESR) supports the statement that New Zealand is experiencing a monoclonal epidemic. While there has always been a background rate caused by other serogroups, and genotypes of group B meningococcal disease – if we remove the epidemic strain from the equation we are left at rates of disease close to pre-epidemic levels.
An epidemic is defined by the Centres for Disease Control in the United States of America as the occurrence of more cases of disease than would normally be expected in a specific place or group of people over a given period of time. The increase in numbers of cases since 1991 is clearly attributable to a single clone of serogroup B Neisseria meningitidis. While not all cases are the epidemic strain of group B meningococcal disease – rates of disease in New Zealand above 3 cases per 100,000 population is clearly attributable to the epidemic strain. Rates in New Zealand in 2004 remain more than 3 times WHO guidelines for epidemic levels of disease.
Meningococcal Gold Rush Authors
Respond:
It is correct to say that “where a isolate
could be taken – approximately 75 percent relate to the
single B:4:P1.7b,4 strain of meningococcal disease.”
So why did the MOH tell Cabinet “The current epidemic has been caused by a single strain of group B meningococcal bacterium” knowing this to be untrue?
- We would expect a Royal
Commission of Inquiry to investigate the misleading use of
statistics evident in this
statement.
But not all notified cases of meningococcal disease are accurate diagnoses. The ESR analysis reveals that between 10 percent and 25 percent of notified meningococcal disease cases are falsely diagnosed. [63]
It is also well known that PCR tests can over-diagnose disease due to the fact that the normal immunological response is to digest bacteria normally present in mucosal tissue. The DNA fragments are part of a normal immune response and they can be detected by PCR and wrongly used to diagnose disease.
If 75 percent of types cases are one type and the other 25 percent are other types then by definition the spread of types rules out a monoclonal or single-strain epidemic. It would be scientifically justifiable to claim that the epidemic has been dominated by a single strain. But it is pseudo-science to state that the epidemic is monoclonal as the MOH does.
The statement “The increase in numbers of cases since 1991 is clearly attributable to a single clone of serogroup B Neisseria meningitides” is patently false.
According to the MOH there have been 5,658 cases of MD and 25 percent are caused by other types.
This equates to 1,414 cases due to other types… so the increase has not been “clearly attributable to a single clone of serogroup B Neisseria meningitides.”
Rates of confirmed cases of the epidemic strain of meningococcal disease are approximately 1.5 times the WHO definition, not the exaggerated 3 times as claimed by the MOH spin doctor.
It is pseudo-science to use all cases to justify a strain specific vaccine.
22. The Ministry of Health says:
Fallacies: Claims that between 10 and 25 percent of notified cases are likely to have been falsely diagnosed and that Cabinet was falsely told that, "the current epidemic has been caused by a single strain of group B meningococcal bacterium”.Facts: The statement that 10-25% of notified cases been falsely diagnosed was not stated in the 2002 Meningococcal Disease Epidemiology report, nor validated by the lead author of the report, Dr Diana Martin.
This information was advised to Ron Law in November 2004 in response to his claims written to the Office of Controller and Auditor-General – yet he has chosen to repeat the statement. ESR advises that there is also a misconception in this statement as only those cases that have been confirmed can be a part of the determination of the impact of the epidemic strain. We cannot define which meningococcus caused the illness in those cases not confirmed by the isolation of a meningococcus or meningococcal DNA. We can only best guess based on the trends of those that are confirmed.
The statistics for the epidemic has been determined on substantiated and robust information. All cases of meningococcal disease in New Zealand are notified on suspicion and are variously confirmed or denotified if an alternative cause is found for the clinical presentations.
For those cases confirmed, evidence is provided in the form of an isolation of a meningococcus from an otherwise sterile site (blood and cerebrospinal fluid (CSF) in particular), the isolation of meningococcal DNA from the blood or CSF, or gram negative diplococci seen in a CSF.
Those cases not confirmed remain in the system on the basis of their clinical description. It should be noted that the symptoms of meningococcal disease can be confused with other conditions and therefore we would expect that a few of the unconfirmed cases may not in fact have a meningococcal origin. In the same way, some cases of mild presentations of meningococcal disease have probably gone undetected and may never therefore have been counted in the total of meningococcal disease cases.
Meningococcal Gold Rush Authors
Respond:
This is another example of the MOH
falsifying facts. The Meningococcal Gold Rush stated,
“Even this figure is in question as a MOH commissioned
report suggests that between 10 and 25 percent of notified
cases are likely to have been falsely diagnosed.[31]” [31] The Meningococcal
Gold Rush report never said that this figure was stated
in the ESR report.
The MOH and the ESR should, however, read their own reports.
Page 9 of the 2002 annual report on the MOH website states;
“Given that 74.1% of notified cases (413/557) are laboratory confirmed, the proportion of true cases is likely to be high. A reasonable estimate would be 90% or 501 in 2002….
The PPV has not been calculated for meningococcal disease. Given that 74.1% of cases (413/557) were laboratory confirmed, this proportion gives a lower-bound estimated for the PPV.”
Based on this statement the ESR clearly believes that between 10% and 25.9% of notifications of meningococcal disease are falsely diagnosed.
Are they now trying to rewrite their 2002 report?
The Ministry of Health sums up its approach to many facets of its MeNZB™ vaccine programme when it says… “We can only best guess…”
The majority of their case and PR spin is based on guess-work as we rightly raised in our Meningococcal Gold Rush and MeNZB™ Quick Guide documents.
- We would expect
a Royal Commission of Inquiry to investigate why a $200
million mass vaccination of 1.15 million otherwise healthy
children was based on pseudo-science and guess
work.
The MOH says, “All cases of meningococcal disease in New Zealand are notified on suspicion and are variously confirmed or denotified if an alternative cause is found for the clinical presentations.”
This is at odds with reality. If 25 percent of notified cases have not been confirmed, as acknowledged by the MOH, then why haven’t they been denotified? By the MOH’s own admission, approximately 1,400 ‘suspicious’ cases are being defined as actual cases when they may not be. In other words, the MOH is masquerading guesswork as fact--again.
23. The Ministry of Health says:
Fallacy: Claims about the death rate and that the epidemic strain targeted by the MeNZB(tm) vaccine has averaged a death rate of just 1.4 percent for the past two years.Facts: This presentation of data represents poor epidemiological practice. Because not all cases can be confirmed by isolate or PCR results to define the sero-subgroup it is inappropriate to present the information as above. It is better to present on five year aggregated rates (where as Mr. Law has selectively chosen the two years that have show the widest contrast). The rate of 1.4% does not take into account epidemic cases that are ‘probable’ cases where an isolate or PCR test could not confirm that they are epidemic strains, or cases that are confirmed as serogroup B, but further typing not possible.
Meningococcal Gold Rush Authors
Respond:
Good science deals in fact… not guess
work. Guesswork and assumptions should be declared as such,
and not masqueraded as fact.
Good policy deals in the most up-to-date information, not out of date historic data.
If all notified 2004 cases are used then 8 deaths per 342 cases gives a death rate in 2004 of 2.3 percent. If all the deaths and all of the cases were of the MenBe type then the theoretical maximum death rate would be 2.3 percent.
Given that we know that only 4 of the deaths were due to the epidemic strain, and 73 percent of confirmed cases were due to the epidemic strain then we have a death rate of due to the MenBe strain of 1.6 percent.
We are happy to adjust the 1.4 percent death rate claimed to 1.6 percent based on the Ministry of Health’s argument as it highlights that we are fully correct to claim that the Ministry of Health’s 4 percent death rate claim is historic and a gross exaggeration with regard to MenBe.
As previously noted, the MOH has recently given the Meningitis Trust totally false statistics to promote their public campaign. The MOH claimed;
Did you know?
Of 100 children who get Meningococcal disease:
- 100 will spend from 2 - 50 days in hospital.
- 5 will die.
- 5 - 20 will develop severe brain damage or deafness. They may lose limbs or be left with damaged skin that needs extensive skin grafts.
- 25 will be left with long-term learning or behavioural difficulties.
- 60 will survive unharmed.
The true death rate for New Zealand age groups between 1999 and 2003 are as follows. [64]
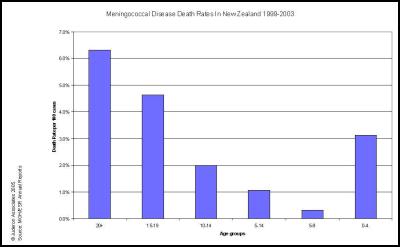
Click for big version
Disability statistics are nothing like those claimed by the MOH. The MOH figures are pure fabrication in the New Zealand context and have nothing to do with New Zealand meningococcal disease.
Again, as discussed previously, we take some satisfaction in the Minister of Health contradicting her officials [who presumably wrote the answer to the parliamentary question for the minister] in her reply to Green MP Sue Kedgley’s Parliamentary Question 06029 (2005).
It’s worth repeating as it shows that it is the Ministry of Health, not us, whose “presentation of data represents poor epidemiological practice,” to quote the Ministry.
The Minister of Health said, “It is well documented in epidemiological literature that the case-fatality rate for serogroup B meningococcal disease is between 2 and 4 times less than other meningococcal disease serogroups that have been reported in New Zealand.” [65]
We knew that, the Minister herself knew that; so why didn’t the Ministry of Health? Perhaps they did?
As discussed earlier, the death rate has been falling since 2001 and the case fatality rate has declined every year but one for the past six years. The last time the death rate was at 4 percent was between 1999 and 2000. [66]
As an aside, we note at mid May 2005 there have been 5 deaths to date. No a single one of those would have been prevented by the MeNZB™ vaccination campaign.
24. The Ministry of Health says:
Fallacies: Claims that the Ministry’s meningococcal vaccine program director, Dr Jane O'Hallahan gave incorrect information to the Seventh Annual Conference on Vaccine Research in Virginia, USA in May 2004.Facts: The information in the presentation was correct in relation to the epidemic claiming up to one life every two weeks in a nation of four million. In 2001 New Zealand had 26 deaths reported. The presentation referred to “up to one life every two weeks”.
Meningococcal Gold Rush
Authors Respond:
The presentation was exaggerated
and would appear to have been deliberately misleading. In
2004 Dr O’Hallahan had 2003 data that showed that the death
rate was half of that quoted. Even allowing for print
lead-time the death rate was somewhat less than the picture
painted.
The choice of the words ‘up to’ could only have been selected to exaggerate the current situation. The number of deaths due to the epidemic strain of meningococcal disease was a fraction of that portrayed.
25. The Ministry of Health says:
Fallacies: Claims that the Ministry of Health mislead people about the vaccine causing herd immunity. And that there is also no compelling scientific evidence that the Cuban vaccination program conferred herd immunity.Facts: The principles behind “herd immunity” remains the same. Herd immunity is not an explicit goal of the Meningococcal B Immunisation Programme – but the likelihood could only be achieved on a national approach, which was the preferred option of the Ministry of Health when information was presented to Cabinet.
The decline in rates of meningococcal disease in Cuba occurred at the same time their group B OMV vaccine was introduced in 1988 and would have contributed to the dramatic overall decline in rates of meningococcal disease by 1989. The decline between 1988 and 1989 could not be interpreted as a ‘natural’ decline
The wording used by the authors are misinterpreting the actual statement in the paper; until data is available then no definitive statement on herd immunity would be made about the MeNZBtm vaccine. The Programme does not rely on herd immunity to control the epidemic.
Meningococcal Gold Rush Authors
Respond:
The MOH funded studies show that carriage
of meningococcal bacteria in about 20 percent of the
population at any one time is not affected by the MeNZB™
vaccine and therefore herd protection is most unlikely. This
has been acknowledged by Professor Diana Lennon, the former
MeNZB™ principal researcher and her team. [67]
The decline of meningococcal disease in Cuba was in free fall decline prior to introduction to their meningococcal vaccine. [68] The pattern is very similar to that occurring in Counties Manukau, Bay of Plenty, Waikato, Northland and other areas... before the vaccine was introduced. , [69] [70]
Parents are being told that they must get their children vaccinated otherwise they are putting other children at risk. Newspaper editorials such as that which appeared in the Hawkes Bay Today on 18 April make claims assuming herd immunity. The MoH are happy to conveniently let such comments go uncorrected. Even specialists are saying that unvaccinated children should be kept away from school–falsely implying herd effect; this appears to be using moral black-mail which is anathema to good informed consent practice.
26. The Ministry of Health says:
Fallacy: Claims about influencing “political will'.Facts: Analysis of community and political attitudes towards any policy decision is good practice. It would be poor practice to not inform a decision making body about community expectations amongst other decision variables, as the funding for the MVS project came from taxpayer funding.
The “paper” discussed was not a formal paper presented to the conference, but a passive poster for the conference. The abstract for the poster simply stated: New Zealand is embarking on an ambitious programme to introduce a strain-specific group B meningococcal vaccine in the context of extraordinary levels of meningococcal disease. New Zealand is in the 12th year of a large and widespread epidemic of group B meningococcal disease (2001: 17.4/100,000) increasingly dominated by a single subtype (P1.7b,4) causing 85 percent of disease in 2000.
Overall, New Zealand children under 5 have a 1 in 330 risk of contracting this disease. It is expected that without a vaccine intervention this outbreak will continue for at least another ten years with potentially 4000 more cases, 200 deaths and perhaps 600 permanently disabled in some way mostly in the under 20 year olds. It has been estimated that in societal costs the meningococcal epidemic to date has cost $500 million NZ dollars. Direct costs to the health sector have been estimated at $220 million NZ dollars.
A ‘tailor-made' vaccine approach ie fitting the vaccine strain to match the outbreak strain has been recognised internationally and nationally as the correct approach, when the highly clonal serogroup B outbreak in New Zealand is dominated by a single strain.
The Government has committed significant funding to a group B mass vaccination campaign targeting all under 20 year olds in New Zealand. A cost benefit analysis provided justification for this intervention. A contract has now been signed with Chiron Corporation to supply for clinical trials and a mass vaccination campaign a New Zealand epidemic strain meningococcal vaccine. Chiron Vaccines is working with Norway's National Institute of Public Health (NIPH). New Zealand's approach has been to form a consortium of the Ministry of Health, Chiron Vaccines and a research team lead from the University of Auckland, to manage the clinical trials and roll out.
Meningococcal Gold Rush Authors
Respond:
Whilst analysis of community and political
attitudes towards any policy decision is good practice,
brazen “harnessing” of political will by officials is
not.
The Ministry has conveniently left the title of the abstract: "How to harness the political will and implement an OMV vaccine solution to combat a devastating epidemic."
- This ‘harnessing of
political will’ by government officials should be
investigated by the Royal Commission of
Inquiry.
27. The Ministry of Health says:
Fallacy: Claims about the choice of Chiron Corporation as the manufacturer of the New Zealand vaccine.Facts: Four manufacturers were considered by an independent selection to the Ministry of Health. The selection of Chiron as the preferred provider of a strain specific vaccine was based on a range of criteria assessed by this panel. It is incorrect that Chiron managed the MeNZBtm clinical trials as this was done jointly by the Ministry of Health, Auckland University and Chiron Vaccines along with expert advisors in other relevant fields of science, epidemiology, statistics, and public health.
The decision of purchase of the vaccine was quite separate from the management of the clinical trials. Any funding decision on vaccine purchase remains with the Crown. The Cabinet paper does not state that Chiron would only produce the vaccine if they got the contracts to both manage the trials and supply the vaccine for the clinical trials and the roll-out.
Meningococcal Gold Rush
Authors Respond:
The Ministry of Health would be
advised to re-read the Cabinet papers, in particular
paragraphs 3, 11 and 30 of the memorandum and bullet point 7
on page 26.
The following statements are juxtapositioned. The statement, “Chiron was only prepared to become involved, if New Zealand committed to a comprehensive project, covering both clinical trials and supply of the vaccine for a mass vaccination campaign.” And the statement “The clinical trials will be managed by the Meningococcal Management Team, a group consisting of the Ministry of Health, Chiron Corporation and the University of Auckland.”
Any reasonable person reading the whole Cabinet paper would be left in no doubt that the Cabinet was given Hobson’s choice; all or nothing. If Chiron didn’t get their way they would pack up and go home.
As if the above [moral] blackmail is not enough, officials include a final paragraph (para 87) prior to their recommendations to Cabinet;
“Large public and media reaction is expected if campaign does not go ahead.”
-We
challenge the Ministry of Health to release the record of
the so-called independent selection panel that selected
Chiron as the sole supplier and to release details of the
processes used to appoint this independent
panel.
We note that the MOH now claims in its response to the MeNZB™ Quick Guide that four tenders were assessed by the MOH. These should be released, especially as the price Chiron was paid was negotiated after the tenders closed and after they had been put in a privileged bargaining position.
28. The Ministry of Health says:
Fallacy: Claims that the Ministry of Health discarded a competitor of Chiron's from consideration as a supplier of a meningococcal B vaccine because their vaccine was a B/C combo.Facts: The RFP for a vaccine supply requested a monovalent vaccine be developed, given ethical considerations of introducing product into people’s body that was not required. The vaccine considered for New Zealand was not a B/C vaccine, but a bi-valent group B vaccine. The example of Cuba was directly relevant in how New Zealand would plan for a mass immunisation programme.
Meningococcal Gold Rush Authors
Respond:
The Ministry of Health would be advised to
re-read the MAAC VSC minutes of 5 April 2004, and the
Cabinet memorandum and associated papers of November
2001.
The former states that the Cuban manufacturers were excluded because of B/C make up of their vaccine and both papers refer to Cuban data (based on a B/C vaccine) to justify a B only vaccine.
29. The Ministry of Health says:
Fallacy: Claims about the control used in the clinical trials.Facts: It is standard practice to use other vaccines as control arms in clinical trials when they are double-blinded controlled studies as were the case with the MeNZBtm clinical trials. Ethically it was agreed that it was better providing children in the control arm of the study with a vaccine benefit for a disease recorded in New Zealand, even though it was not against the epidemic strain of meningococcal disease.
Meningococcal Gold Rush Authors
Respond:
The MeNZB™ trials were not double blinded
as claimed.
- We would expect a Royal
Commission of Inquiry to investigate why the Ministry of
Health has knowingly stated that the MeNZB™ trials were
double-blinded and controlled when they weren’t.
In fact the trials undertaken are not those referred to in the 2001 Cabinet papers.
Cabinet was told that rollout was subject to satisfactory vaccine performance in “a series of clinical trials proposed to assess the safety, immunogenicity and efficacy of the candidate vaccine in up to 5,500 individuals.” [our emphasis]
We now note that the MOH are claiming that the effectiveness of the vaccine will be determined after the vaccination program is complete. This is because the MeNZB™ campaign in Counties Manukau and other areas has made no measurable indent on the overall impact of meningococcal disease. In other words, the Ministry of Health knows already that the vaccine is not decreasing the burden of disease in New Zealand, and yet it is continuing to bully parents into signing their children up to this mass medical experiment.
The efficacy studies were never undertaken and according to the MOH, “approximately 1,700” individuals were involved in what studies were undertaken, less than a third of the number given to Cabinet.
- Assuming funding was based on
studies of 5,500 individuals we would expect a Royal
Commission of Inquiry to investigate what happened to the
millions of research dollars approved by Cabinet and
destined for the other 3,800
individuals.
The claim that it would be more ethical to use another vaccine rather than a placebo is preposterous, especially given that unlicensed drugs were used. On this basis no gold standard placebo would ever be used in any clinical trial. Why were commonly used vaccines also used? Was this an attempt to vaccinate children by stealth?
- We would expect
a Royal Commission of Inquiry to investigate why Chiron
Corp’s unlicensed Menjugate Men C was used in one of the
trials?
The MOH statement that follows is a spin-doctor masterpiece. “Ethically it was agreed that it was better providing children in the control arm of the study with a vaccine benefit for a disease recorded in New Zealand, even though it was not against the epidemic strain of meningococcal disease.”
If, as the Ministry of Health claims, meningococcal C is not prevalent in New Zealand at sufficient levels to warrant use of at least two already fully licensed vaccines that target meningococcal C, what protection would an unlicensed MenC vaccine provide 6-10 week old babies?
We are of the view that use of the Menjugate vaccine was simply to provide Chiron with a free clinical trial of their vaccine.
- We would expect a Royal Commission of Inquiry
to investigate whether those tests are being used by Chiron
Corp to assist with global licensing of Menjugate
C?- We would expect a Royal
Commission of Inquiry to investigate whether Chiron Corp
tested antibody responses to their Menjugate C vaccine in NZ
children?
Also, were the subjects in the trials already vaccinated with the various vaccines used as controls? If so, what benefits would be gained?
30. The Ministry of Health says:
Fallacy: Claims that Chiron was undertaking unapproved trials of a vaccine that was not licensed for use in New Zealand nor the USA.Facts: All trials ran in NZ had to go through SCOTT and ethic committees before the study could commence. The reason that you undertake clinical trials is to seek a licence. Menjugate is licensed for use in 26 countries around the world including the UK, Canada, Australia and through out Europe and Latin America.
Meningococcal Gold Rush Authors
Respond:
Apart from the fact that the MOH has
misrepresented our claim that, “Were parents and guardians
aware that Chiron was undertaking what would appear to
be unapproved trials of a vaccine that is not licensed
for use in New Zealand nor the USA?” the fact remains,
Menjugate C is not a licensed medicine in New Zealand.
If Menjugate C is so good, why hasn’t it been licensed here? If it is so good, why has Chiron withdrawn its application for a licence in the USA, its home country?
Were parents or participants in the trial told that a ‘placebo’ was in fact an unlicensed Menjugate C vaccine?
31. The Ministry of Health says:
Fallacy: Claims that the meningococcal B bacterium is uniquely resistant to vaccination.Facts: You don’t vaccinate bacteria, you vaccinate people. Group B meningococcal bacteria have a highly variable capsular surface, which has made it difficult to develop traditional cell-surface based vaccines.
It is for this reason that genomic-based vaccines targeting group B meningococcal disease are being looked at to circumvent issues to do with cell-surface variability. As a result of these problems, serogroup B vaccines that utilise the outer membrane protein (OMP) layer (particularly Part 1 or PorA epitope) have been developed. These vaccines have proved to be immunogenic. However, OMP vaccines have to be developed for each strain; it is not possible to develop a single strain OMP vaccine for all serogroup B meningococcal organisms. In New Zealand’s case the OMP vaccine will need to be specific for the B:4:P1.7b,4 strain.
While a ‘genomic’ vaccine is not yet available, results from the clinical trials undertaken between 2002-2004 have shown the MeNZBtm vaccine to generate high levels of antibody activity in all age groups in New Zealand’s who received the strain specific OMV vaccine.
In addition, most other countries will experience multiple sub-strains of meningococcal bacteria causing invasive meningococcal disease. Because group B meningococcal vaccines need to be strain-specific, a mass immunisation public health intervention will not control the levels of disease. New Zealand has experienced a very stable monoclonal epidemic of one strain of meningococcal disease. The technology was available and the opportunity was there to control the current epidemic of meningococcal disease in New Zealand.
Meningococcal Gold Rush Authors
Respond:
We don’t mind admitting that the statement
“meningococcal B bacterium is uniquely resistant to
vaccination” could have been written more clearly.
The reason group B vaccines have not been used in the past relates the fact that antibodies generated against group B polysaccharide vaccines affect the brain; hence the absence of B polysaccharide vaccines.
Most other countries have a predominance of group B meningococcal disease, and do not vaccinate against this strain because there is no proven effective vaccine. Even Norway has rejected their own ‘tailor-made’ OMP vaccine because it wasn’t effective enough; yet the MOH used their data to guess efficacy for MeNZB™. It’s worth repeating here that even one of the MOH’s outside advisors, Professor Cartwright from the UK says that OMV meningococcal B vaccines are “ineffective in infants.” [71]
The MOH says that clinical trials undertaken between 2002-2004 have shown the MeNZBtm vaccine to generate high levels of antibody activity in all age groups in New Zealand’s who received the strain specific OMV vaccine.
Is the MOH implying that a 55 percent response rate for under 6-month olds is high?
32. The Ministry of Health says:
Fallacy: Claims over the use of bridging information and that this was akin to Vioxx being approved based on Celebrex data; that because there was only a minor difference in the two drugs, the adverse effects and benefits would be the same. The fact that the Norwegian vaccine designed for their epidemic was never approved for routine use in its own country because it wasn't effective enough seems to have slipped from memory here.Facts: Each part of a clinical trial is designed to answer questions about a new product that are either unknown or require clarification. The use of bridging information - or designing bridging trials, to obtain this information - is a standard process to help test a single hypothesis or set of hypotheses about a new product that is closely related to an original or “parent” product. The design of the New Zealand MeNZBtm clinical trials was heavily influenced by existing information that was available on the parent MenBVactm vaccine, developed by the Norwegian Institute of Public Health, and other similar group B meningococcal vaccines using the same outer membrane vesicle (OMV) technology.
While the MenBVactm vaccine targeted a different antigen to the New Zealand epidemic strain, the MeNZBtm vaccine was manufactured using the same technology and ingredients and was used as a control arm in the Phase I/II MeNZBtm clinical studies. The information pertaining to the safety profile of the MenBVactm vaccine and the MeNZBtm clinical trials was sent to the Medsafe for assessment as part of the overall licensure process. A phase III clinical trial was agreed by international experts and the New Zealand clinical trial management team to not answer additional questions that were already answered. In addition this would have delayed the introduction of the MeNZBtm vaccine and ability to control the current epidemic sooner.
Ron Law and Barbara Sumner-Burstyn are not comparing apples with apples. NIPH officials have stated that the decision not to introduce the Norwegian MenBVactm vaccine was made in the 19th year of their epidemic because there was evidence that the epidemic was waning naturally and a vaccine programme would not have been cost beneficial. It was noted in later studies that a 3 dose schedule greatly improved the antibody response
Meningococcal Gold Rush Authors
Respond:
Of course, the big piece of the jigsaw
puzzle here is that there was no efficacy data to show that
the MeNZB™ vaccine would actually work. The MOH now claims,
“A phase III clinical trial was agreed by international
experts and the New Zealand clinical trial management team
to not answer additional questions that were already
answered.”
We are puzzled by this illogical advice. The Norwegian study answered the question, “Does the vaccine work?” In the New Zealand context, the Norwegian conclusion was, “It works a bit, but not enough to spend $200 million of precious taxpayers money that the Treasury said would give a better social dividend elsewhere, like improved housing conditions, and did not warrant exposing 1.15 million otherwise healthy children to unknown risks and a false sense of security.”
As noted earlier, the NIPH stated in their published paper in The Lancet that the vaccine did not have enough efficacy to warrant mass use. [72] Regardless, as was occurring in Norway, the epidemic is waning naturally in New Zealand and the current vaccine programme is not cost beneficial.
As to comparing apples; the difference between Vioxx, Celebrex and Bextra is chemically minute - but using MOH logic it should be possible to bridge information from one to the other. How bizarre for a regulator to make such an assumption, especially at a time when Vioxx and Bextra have been recalled and the others have major warnings on packets and package inserts?
33. The Ministry of Health says:
Fallacy: Claims that only through phase III testing was it proven that Vioxx and Celebrex are lethal drugs responsible for tens of thousands of deaths in the US and several hundred at least in Australasia.Facts: This was not picked up in phase III testing. This was assessed in Post-marketing surveillance. Phase III testing was not favoured by international review of the New Zealand strategy because of the amount of relevant data already available and the context of the epidemic levels of disease.
Meningococcal Gold Rush Authors
Respond:
The Ministry of Health is wrong, again.
The Vioxx study was a phase III trial. According to Merck, the APPROVe (Adenomatous Polyp Prevention on VIOXX) trial was a multi-centre, randomized, placebo-controlled, double-blind study to determine the effect of long-term treatment with VIOXX on the recurrence of neoplastic polyps of the large bowel in patients with a history of colorectal adenoma. The trial enrolled 2,600 patients and compared VIOXX 25 mg to placebo. The trial began enrolment in 2000.
According to Merck, the study was stopped early because three-year data showed an increased relative risk for confirmed cardiovascular events, such as heart attack and stroke, beginning after 18 months of treatment in the patients taking VIOXX compared to those taking placebo. The results for the first 18 months of the APPROVe study did not show any increased risk of confirmed cardiovascular events on VIOXX.
People with expertise in pharmaco-vigilance and recent recalls due to unacceptable adverse effects of drugs would have known this.
If the MOH can not get basic facts like this correct when claiming others are wrong then what faith can New Zealanders have that the MOH statements about MeNZB™ are correct?
34. The Ministry of Health says:
Fallacy: Claims questioning the roles of New Zealand researchers and medical regulators.Facts: The ability to present results to colleagues is a critical means of getting peer review and ensuring that the scientific and medical community are constantly learning to ensure that the best possible health results can be achieved and that the process followed and results are validated.
The publishing of proceedings from such conferences allows the dissemination of results for further peer review. It was also essential that the results were presented by a New Zealand representative given the Meningococcal Vaccine Strategy is a New Zealand driven project. The scrutiny provided by international peer reviewers at such conferences allows immediate feedback that is invaluable. All invitations to attend conferences are reviewed by officials and attendance is based on the merit and audience involved. Not all conferences are attended, as the delivery of the programme in New Zealand is the prime focus of the person discussed above.
Meningococcal Gold Rush Authors
Respond:
Preaching to the converted is not peer
review.
The incestuous nature of researchers, advisors, policy makers, regulators and associates is highlighted by the multi-authorship of many papers: Who is left to peer review and provide an impartial perspective?
People usually apply [and pay fees] to attend conferences.
It is worth noting that the authors of the so-called Proceedings of the Meningococcal Vaccine Strategy World Health Organization Satellite Meeting, 10 March 2004, Auckland, New Zealand are the following;
Kerry Sexton, Diana Lennon, Philipp Oster, Ingeborg Aaberge, Diana Martin, Stewart Reid, Sharon Wong, Jane O’Hallahan.
Kerry Sexton is a
Public Health Medicine Registrar, Meningococcal Vaccine
Strategy, Ministry of Health,
Diana Lennon was,
until recently, Principal Researcher of MeNZB™ and is
Professor of Population Health of Children and Youth,
University of Auckland,
Philipp Oster is
Associate Director, Clinical Research and Medical Affairs,
Chiron Vaccines, Siena, Italy;
Ingeborg Aaberge
is Department Director, Department of Airborne Infections,
Division of Infectious Disease Control, Norwegian Institute
of Public Health, Oslo, Norway;
Diana Martin is
Principal Scientist, Institute of Environmental Science and
Research Limited (ESR), Porirua;
Stewart Reid is
a family physician who has been involved in vaccinology for
the last 22 years; has been a member of the committee which
advises the New Zealand Government on immunisation policy
since 1980 and has chaired the committee for much of that
time; has been involved in the writing and editing of all
three editions of the New Zealand Immunisation Handbook;
i.e. as a member of the licensing MAAC vaccine subcommittee
and makes guest appearances at the full MAAC committee of
which he is not a member, is as permanent advisor to the
Meningococcal Management Team, a consortium of the New
Zealand Ministry of Health, Chiron and the University of
Auckland, and, according to Clinical Trial Application for
study V60P4 is the principal investigator for one of two
trials of the Chiron made MeNZB™ vaccine.
Sharon Wong
is a Clinical Research Fellow, University of Auckland,
and a sub-investigator of Chiron’s Clinical Trial V60P4 (as
well as other MeNZB™ trials)
Jane O’Hallahan, is
Director, Meningococcal Vaccine Strategy, Ministry of
Health, Wellington
- Is
this the group who independently endorsed the MeNZB™ vaccine
on behalf of the World Health
Organisation?- We ask the
important question. Why are there no independent researchers
who have no conflict of interest as to whether the MeNZB™
vaccine goes ahead or not included in this who’s who of
WHO’s advisors…? They are all pro-MeNZB™.
In other words: The foxes appear to be in charge of the henhouse, vetting themselves and recommending their own work.
Where is the transparency?
Why were only some of these competing interests declared in this NZMJ paper?
Were these the same independent advisors who represented the WHO in their positive advice to Cabinet?
35. The Ministry of Health says:
Fallacy: Claims of potentially dangerous conflicts of interest extend to the MeNZB(tm) adverse event monitoring system and that this has resulted in discounting deaths following meningococcal vaccination as due to 'accident' or 'other unrelated illness'.Facts: All deaths in vaccinees are comprehensively assessed to establish cause of death. All deaths in vaccinees to date have been clearly due to a cause non-related to MeNZBtm vaccination, eg car accidents. New Zealand has been applauded for rigorous safety monitoring system that has been established to monitor post-licensure of the MeNZBtm vaccine. The New Zealand model includes assessment of all adverse events following immunisation (including any potential new AEFI signals not previously associated with other vaccines). Methods include assessment of admissions to hospital and emergency department consultations (under 20 years of age).
Approximately 100,000 vaccinees under 5 years of age and 100,000 vaccinees over 5 years of age will be monitored through Auckland, Middlemore and Whangarei hospitals. Ongoing data matching of hospital admission and discharge data and immunisation data will occur so that any event or disease that may be of possible concern can be linked back to immunisation status. This gives the ability to identify new AEFI signals for further review.
In addition any health professional or member of the public may report any suspect adverse event to CARM. The Independent Safety Monitoring Board (ISMB) is run by the Health Research Council, and is separate from both the Ministry of Health who plan and promote the Meningococcal B Immunisation Programme and Chiron Vaccines, the MeNZBtm vaccine manufacturer.
Neither the Ministry of Health nor Chiron Vaccines are involved in any decision making of the ISMB. The ISMB assesses the collection of safety data and can at any time recommend the cessation of the Meningococcal B Immunisation Programme to the Ministry of Health. Safety of the MeNZBtm vaccine has been paramount to the entire strategy.
Meningococcal Gold Rush Authors
Respond:
All deaths in vaccinees are
comprehensively assessed to establish cause of death.
The MOH states that the Independent Safety Monitoring Board (ISMB) is run by the Health Research Council, and is separate from both the Ministry of Health who plan and promote the Meningococcal B Immunisation Programme and Chiron Vaccines, the MeNZBtm vaccine manufacturer.
The MOH initiated the establishment of a safety monitoring board outside of the Health Research Council’s standing drug safety monitoring board committee members. Why did the Ministry of Health interfere in due process? Why have colleagues and associates of MeNZB™ researchers and advisors been placed on the so-called independent safety monitoring board?
The MOH claims that all [approximately 40] deaths in vaccinees are comprehensively assessed to establish cause of death.
This appears to be more fiction by the MOH spin-doctors. If each death had been “comprehensively assessed to establish cause of death” then each cause of death would have been classified using internationally agreed WHO classifications such as those displayed on the Ministry of Health’s own website. [73]
International best practice classifies each death and adverse event report as: definite, probable, possible, unlikely, or more information needed to determine causality.
- We
would expect a Royal Commission of Inquiry to investigate
why has use of international best practice, and the Ministry
of Health’s own procedures have been bypassed in the case of
assessment of causality in deaths and adverse events
associated with MeNZB™?
The ISMB is supposed to be able to recommend cessation at any time. However the multiple conflicts of interest exhibited by members of the ISMB make this scenario most unlikely.
These conflicts include:
A member co-authored (with a MeNZB™advisor/researcher) a recommendation advising Maori health care providers to agree with the meningococcal vaccine. This same member is a colleague in the same department as a key meningococcal researcher. This same member recently [jointly] received a multi-million dollar grant from the HRC.
A member is a co-professor in the same department as the principal MeNZB™ researcher. This same member recently received a multi-hundred thousand dollar grant from the HRC to research why some people choose to not use vaccines.
A member has a close relationship with Chiron’s Australian licensee.
- Why have
members of the HRC standing committee on drug safety
monitoring who have pharmaco-vigilence expertise been
replaced by members who don’t?Among
the many others, we would like the following questions
answered by the Royal Commission of Inquiry;
- Have there been cases of
meningococcal disease in fully vaccinated children, such as
a sixteen year old male at Auckland Hospital, who were
treated as if they had meningococcal disease but not
diagnosed with meningococcal disease?
- Were hospital
staff told to keep quiet?
- Was there a death of a
vaccinated baby in Auckland recently who, according to staff
within the system, died of ‘meningococcal-type’ disease? If
so, has this death been
reported?
If it is the ISMB who is assessing adverse reactions, what is the role of the hitherto unheard of Clinical Review Committee chaired by Dr Barry of the Hawkes Bay District Health Board?
We have on file a copy of a power-point presentation used at District Health Board/MOH public meeting in the Hawkes Bay. It shows that the Meningococcal Management Team [including MOH, Chiron and MeNZB™ researchers] has placed a hand chosen committee between the medical records of vaccines who have suffered possible adverse effects and the ISMB. In fact, as discussed earlier, this committee reports to the Meningococcal Vaccine Strategy team within the MOH who analyse data for the ‘Independent’ ISMB.
- Why haven’t adverse reactions been assessed
according to Ministry of Health’s own causality definitions
such as definite, probable, possible, unlikely, definitely
not and more info
needed?- Who pays this hand chosen
group? and what pharmaco-vigilance expertise do they
have?
36. The Ministry of Health says:
Fallacy: Claims of two deaths during the trials.Facts: Ron Law was written to by the Minister of Health in October 2004 where the Minister stated I can confirm that there has been no deaths to date related to the MeNZBtm vaccine. As already stated in the press release put out by the Ministry of Health dated 9 October 2004 “During the same monitoring period there were two deaths in people who had co-incidentally been vaccinated. One programme participant died in an accident and the other died of an illness unrelated to the vaccine.” Both cases were reviewed by the Independent Safety Monitoring Board.
The ISMB has since considered 10 deaths in vaccinees occurring between 19 July to 28 November – of which all had a clear cause of death not attributable to the MeNZBtm vaccine.
Meningococcal Gold Rush
Authors Respond:
It is a fact that two deaths
occurred during the trials in vaccinees given the MeNZB™
vaccine. Thirty eight more have occurred since. Those deaths
were related to vaccine use, although causality has not been
formally nor independently assessed.
Scientifically valid trials would have included a control group so that the statistical significance of these could be determined. No such control group exists in the MeNZB™ experiments.
It was only by having a double-blind, placebo-controlled group that Vioxx was proven to be dangerous and removed from sale.
The lack of control groups is a fundamental flaw in the design of the MeNZB™ mass experiment.
Based on the latest ISMB report available publicly, 38 previously healthy children have died following MeNZB™ vaccination. The ISMB simply states; “However, the causes of death were trauma (mainly car accidents), or medical conditions, illnesses or infections that explained the death” with no evidence of formal causality assessment. [74]
These dismissive statements follow exactly the same pattern of denial as were vented against whistle-blowers of drugs such as Vioxx, Bextra, Prozac and Aropax prior to analysis independent of the influence of drug companies.
By definition children being vaccinated in NZ are healthy. Healthy children have died shortly after receiving the MeNZB™ vaccine. We challenge the MOH to publicly state how many of these deaths have been assessed using standard pharmaco-vigilance practice as displayed on their Medsafe website. How many have been referred for a Coroner’s inquiry?
For example, the 5 year old in Manukau. That death should be referred to a coroner’s inquiry due to the extreme likelihood that medical error denied the child any chance of receiving life-saving treatment.
For example, we are aware of a baby in Palmerston North dying recently after its first MeNZB™ vaccination.
Why is the MOH only referring to the 10 deaths up to November? Why hasn’t it even referred to the 26 deaths recorded up to the end of February?
For example, we have been informed of a fully vaccinated baby in the Auckland region dying from a “meningococcal-type” illness in recent weeks and staff being told to keep quiet.
These deaths need formal independent inquiries to ascertain cause of death.
37. The Ministry of Health says:
Fallacy: Claims there are ethical questions surrounding the Ministry of Health downloading school rolls into its new National Immunisation Register.Facts: The school role data is not recorded directly on the NIR, but the School Based Vaccination System managed by DHBs. School role data is not kept on the NIR. Immunisation event data is then transferred to the NIR. Legal opinions from both the Ministry of Education and the Ministry of Health supported the release of the school rolls to the Public Health services carrying out the vaccinations. The Ministry of Education’s Schools’ Consultative Committee was briefed. This process was also discussed with both the Office of the Privacy Commissioner and the Office of the Ombudsman.
The Privacy Commissioner advised us that before school rolls can be provided to Public Health Nurses, schools will need to inform parents and children that the rolls will be used in this manner. School newsletters would be an appropriate way to inform parents of this. A letter to all Principals in New Zealand include advice about the use of school roles and the need to inform parents through newsletter regarding the provision of school roles to PHNs (sent 20 February 2003 and 14 July 2004).
Meningococcal Gold Rush Authors
Response:
Modern relational databases enable links
to be made between databases so that a larger virtual
database exists.
We have talked to many parents and are aware of being advised that school rolls were being downloaded into health databases, and none were aware that they had the right to veto their children’s information being downloaded. Letters to schools dated 20 February 2003 and 14 July 2004 have been received under the official information act. They state categorically that school rolls are necessary for the success of the MeNZB™ program and make no mention of parents having the right to object.
This important democratic and privacy right should have been part of the consent form. The MOH have clearly not included that in the consent form to prevent their unique opportunity for an apparently illegal census of every child in New Zealand.
The Privacy Commissioner's requirement that parents and children be notified has been breached. Therefore, we contend that the downloading of student personal details is illegal and should be stopped forthwith.
38. The Ministry of Health says:
Fallacy: Claims that the ideology behind introduction of a new meningococcal vaccine as 'Frightening parents about the consequences of failing to vaccinate their children will most likely be part of the campaign'.Facts: All parents and vaccinees are encouraged to make informed decisions; information brochures and consent forms were given to all parents. Information regarding the disease and the Meningococcal Vaccine (the vaccine) was distributed widely to ensure the delivery of the programme complies with the Code of Health and Disability Consumers’ Rights – the Principles of Child Consents to ensure that all vaccinees or guardians can give consent for receiving the three doses of the vaccine and are able to understand the risks and benefits of the procedure and make an informed choice.
Meningococcal Gold Rush Authors
Respond:
The Code of Health and Disability
Consumers’ Rights – the Principles of Child Consents appears
to have been breached as drugs of an experimental nature,
such as those fast-tracked via section 23 of the medicines
act, require written consent even for those attending
medical practices. We are advised that written consent is
not requested by most GPs.
Parents report they are intimidated into signing the forms. None spoken to were aware there was no legal requirement to even return the consent forms – most report being ordered via their freshly motivated children to return their forms the following day. The Hawkes Bay Today reported that Ministry staff have gone into homes to ‘assist’ parents in signing their forms. Non-return of forms or non-consent has resulted in follow-up calls and visits that many parents have reported as pressured.
There is no rationale other than to intimidate parents and scare young impressionable children by showing them gruesome images and videos as consent forms are handed out. As noted, many children go home crying and begging parents for the vaccines because they do not want to lose limbs and die.
This is in breach of the Nuremberg Code, and the subsequent Helsinki Declaration to which New Zealand is a signatory. Informed consent is described as being free from force, fraud, deceit, duress, over-reaching and other forms of constraint or coercion. It must be made on the basis of adequate information concerning research and all available alternatives.
Evidence of extreme coercion is coming in every day; from children told they would be excluded from school trips, lose house points and detention to bribing with sweets, drinks, pizza, house points, and even going in a draw to win one of 16 i-Pods.
This is grossly wrong.
- We believe that such
practice in our schools is immoral, and illegal as it is in
breach of The Nuremberg Code (to which NZ is a signatory)
and should be investigated by the Royal Commission of
Inquiry.
Every parent spoken to has reported frustration at the lack of information, at the use of glossy PR brochures, and the sense that to question the wisdom of this campaign was to put their child at risk and be an anti-vaccinationist.
Schools, who are being paid to administer the vaccination campaign, are been used as functionaries of the Ministry, surely a breach of their function as educators.
In Conclusion:
The attitude, advice and response of the Ministry of Health to our original investigation report, The Meningococcal Gold Rush, would seem to indicate a culture of incompetence, misleading use of statistics and undisclosed conflicts of interest that is more than worthy of a formal Royal Commission of Inquiry.
This is exacerbated by an entrenched arrogance that would appear to extend even to officials misinforming the Prime Minister, who in February stated that meningococcal B was a virus that had killed more than 200 New Zealanders. [75]
Given that meningococcal B is not a virus, and not all of the deaths were caused by the strain targetted by the MeNZB™ vaccine, even the Prime Minister was given false information by officials. As we have seen frequently, while there is a rush to shut down any critique of this vaccine there is no haste in correcting any statements if they are in favour of the vaccine, no matter how false they are.
The evidence trail suggests that the MeNZB™ vaccination programme has been based around an orchestrated litany of lies and public deceit.
Given the fact that MeNZB™ is an experimental drug, we believe that the circumstances surrounding the vaccination campaign are such that it should be halted forth with and a full Royal Commission of Inquiry established to investigate the issues raised.
The questions raised are too serious to ignore, especially when 1.15 million hither to healthy children are being bullied into being nothing more than guinea-pigs in what may be another unfortunate experiment.
1. http://www.scoop.co.nz/stories/HL0502/S00064.htm, 7 February 2005
2. Since posted at http://www.sumnerburstyn.com/vax/MeNZB-Quick-Guide-332.pdf
3. http://www.scoop.co.nz/stories/GE0505/S00181.htm
4. By the end of week ending June 5 2005, meetings will have been addressed by the authors in Auckland, (4) Hastings, Dannevirke, Napier, Wellington (2), Hamilton (2), Blenheim, Nelson, and Takaka.
5. Eg, Complaint 05/051 - Meningococcal B Vaccine Television Advertisement at http://202.36.205.180/decisions/05/05051.rtf
6. See http://www.answers.com/malfeasance&r=67
7. Correspondence from Auditor General’s office to Ron Law dated 10 March 2005 on file.
8. BFM interview with Dr Jane O’Hallohan, http://y3m.net/files/RussellBrown,JaneOHallahan,2005-02-09.mp3
9. The cost benefit analysis was obtained under the Official Information Act. It was prepared by members of a faculty of Auckland University that stood to gain many millions of dollars in research funds if the project went ahead. One author also acknowledges being affiliated with what appears to be a private consulting firm. Another is the Principal MeNZB™ researcher.
10. http://www.clerk.parliament.govt.nz/Content/Hansard/Final/ FINAL_2004_09_15.htm#_Toc84652997
11. http://www.nzherald.co.nz/index.cfm?ObjectID=3573775
12.
Extracts from; Bjune, Hoiby, Gronnesby, et al, Effect of
outer membrane vesicle vaccine against group B meningococcal
disease in Norway. The Lancet, 1093-1096, 2 Nov, 1991
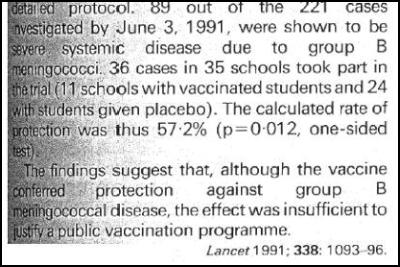

15. Southland Times, 7 May 2005
16. Oster P, Lennon D, O'Hallahan J, Mulholland K, Reid S, Martin D. (2005) MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain., Vaccine. 2005 Mar 18;23(17-18):2191-6.
17. Southland Times, 7 May 2005
18. MAAC Minutes, 5 April 2004
19. Point 29 in MOH MQG response
20. Eg, Complaint 05/051 - Meningococcal B Vaccine Television Advertisement at http://202.36.205.180/decisions/05/05051.rtf
21.
Scan from MAAC vsc 5 April 2004. 
22. Scan from MAAC VSC 5 April
2004 
23. Document on file
24. http://www.inmed.co.uk/lectures/lecture3.ppt
28. See http://www.answers.com/malfeasance&r=67
29. Epidemiology of Menigococcal Disease in New Zealand, 2002. ESR report commissioned for MOH
30. Answer to Parliamentary Question 06029 (2005)
32. See above graph
33. By way of example of the literature: Erickson L, De Wals P, (1998) Complications and sequelae of meningococcal disease in Quebec, Canada, 1990-1994Clin Infect Dis. May;26(5):1159-64. use of vaccination for outbreak control Department of Community Health Sciences of the University of Sherbrooke, Sherbrooke University Hospital Clinical Research Center, Quebec, Canada To study complications and sequelae of serogroup B and C meningococcal disease, a retrospective survey examined the outcome of all culture-proven cases reported in the province of Quebec, Canada, from January 1990 through December 1994 (serogroup B, 167 cases; serogroup C, 304 cases). Data were collected from medical files, postal questionnaires, and telephone interviews. Age groups having the most cases were the 10-19-year age group for serogroup C and the < 1-year age group for serogroup B. Fatality rates were 7 percent for serogroup B and 14 percent for serogroup C disease. Only 3 percent of survivors of serogroup B disease had physical sequelae, compared with 15 percent of survivors of serogroup C disease (skin scars, 12 percent; amputations, 5 percent; hearing loss, 2 percent; renal problems, 1 percent; and other sequelae, 4 percent). These results confirm the gravity of disease caused by serogroup C, serotype 2a Neisseria meningitidis and justify liberal
34. “Meningococcal infections can spread fast and lead to life-threatening diseases, especially meningococcal C disease, which has a higher rate of mortality and sequelae than meningococcal disease caused by other serogroups in developed countries.” Sourced from http://www.chironvaccines.com/company/vaccines_Meningitis_Vaccines.php
35.
(10) N Z Med J. 1989 May 24;102(868):243-5.
The clinical
features of paediatric meningococcal disease Auckland,
1985-87.
Voss L, Lennon D, Sinclair J.
Princess Mary
Hospital, Auckland.
An epidemic of group A meningococcal
disease began in Auckland in May 1985. There were 122
paediatric cases of meningococcal disease in the next 25
months including 98 cases due to group A. The commonest
clinical symptoms were vomiting, headache and photophobia,
while frequent signs included fever, seizures, petechial
rash and meningism or a bulging fontanelle. Complications
were uncommon and included sterile arthritis and prolonged
fever. The majority had disease confirmed by positive blood
or cerebrospinal fluid culture. Significantly fewer positive
cultures were seen in those treated with antibiotics prior
to admission. The overall mortality was 7%. If the acute
illness was survived, the only detected long term sequela
was sensorineural hearing loss seen in 6%. [our
emphasis] A vaccine programme has been undertaken to control
this epidemic. PMID: 2498788 [PubMed - indexed for
MEDLINE]
39. Source: Email received 26 May 2004. MOH confirmed as source of data by Fiona Corbert; personal communication. Following several emails the MOH confirmed the data and then produced a publication with the same factually incorrect information that had been sent to healthcare professionals.
40. MAAC VSC Minutes, 5 April 2004)
41. Chiron’s Application for Approval of a Clinical Trial, V60P4. On file
42. http://www.huttvalleydhb.org.nz/Article.aspx?ID=2889
43. http://www.phaa.net.au/conferences/CairnsConference/Immu%20Speakers.htm …. Dr Stewart Reid, General Practitioner, Ropata Medical Centre, Lower Hutt, New Zealand. Dr Stewart Reid is a family physician who has been involved in vaccinology for the last 22 years. He has been a member of the committee which advises the New Zealand Government on immunisation policy since 1980 and has chaired the committee for much of that time. He has been involved in the writing and editing of all three editions of the New Zealand Immunisation Handbook, as well as a member of the committee in New Zealand which advises the New Zealand licensure authority on the licensure of vaccines. His main involvement in vaccinology at present is as permanent advisor to the Meningococcal Management Team, a consortium of the New Zealand Ministry of Health, Chiron and the University of Auckland.
44. NZMJ., Information for authors. http://www.nzma.org.nz/journal/authors.html
45. PQ 5241 (2003). Dr Lynda Scott to the Minister of Health (28 May 2003): Will New Zealand acquire the intellectual property rights to the meningococcal vaccine that is being trialled? http://publications.clerk.parliament.govt.nz.clients.intergen.net.nz/ QuestionsForWrittenAnswerSearch.aspx]
46. [PQ 5241 (2003). Dr Lynda Scott to the Minister of Health (28 May 2003): Will New Zealand acquire the intellectual property rights to the meningococcal vaccine that is being trialled? http://publications.clerk.parliament.govt.nz.clients.intergen.net.nz/ QuestionsForWrittenAnswerSearch.aspx]
47. Chiron’s Application for Approval of a Clinical Trial, V60P4. On file
48. Document on file.
49. See also http://www.moh.govt.nz/moh.nsf/wpg_index/ Links-Meningococcal%2BVaccine%2BStrategy%2B-%2BKey%2BAgencies&e=10313; http://www.google.co.nz/url?sa=U&start=2&q= http://www.moh.govt.nz/moh.nsf/0/ c8a29f4bd4127992cc257000000be804%3FOpenDocument&e=10313
51. http://www.nzherald.co.nz/index.cfm?ObjectID=3577033
52. http://query.nytimes.com/gst/health/articlepage.html? res=9E02E3DF1E3EF93BA1575BC0A9629C8B63
53. eg, Paras, 9, 10, 29 & 54 Memorandum to Cabinet, Nov 2001
54. http://www.nzma.org.nz/journal/117-1200/1015/
55. Epidemiology of Meningococcal Disease in New Zealand, May 2004. MOH/ESR Publication
56. eg; http://www.immune.org.nz/site_resources/Professionals/ Old%%20newsletters/ImmNuZ_-_May_2005.pdf,
57. see http://www.scoop.co.nz/stories/GE0505/S00094.htm
58. Milne, Evers, Ashton, Lennon, (2001) An Economic Evaluation of Vaccination Against Meningococcal Disease, Auckland University. A report prepared for the MOH
59. http://www.immune.org.nz/print.asp?t=736
60. Memorandum to Cabinet, Nov 2001 and Treasury assessment on file.
61. Those interested should contact the student’s supervisor to verify the facts, http://www.econ.canterbury.ac.nz/people/fountain.shtml
62. Paragraph 1, Cabinet Memorandum, 2001
63. Epidemiology of Meningococcal Disease in New Zealand, 2002 Annual Report
64. Epidemiology of Meningococcal Disease in New Zealand, 2003 Annual Report
65. Answer to Parliamentary Question 06029 (2005)
66. 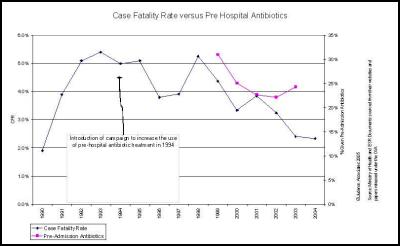
Click for big
version
Case Fatality Rates for All Types of
Meningococcal Disease
67. http://www.google.co.nz/url? sa=U&start=2&q=http://www.nzma.org.nz/journal/117-1200/1026/&e=10313
71. http://www.inmed.co.uk/lectures/lecture3.ppt
72.
Extracts from; Bjune, Hoiby, Gronnesby, et al, Effect of
outer membrane vesicle vaccine against group B meningococcal
disease in Norway. The Lancet, 1093-1096, 2 Nov, 1991,


73. http://www.medsafe.govt.nz/Profs/adverse/causality.htm Causality Assessment of Suspected Adverse Medicine Reactions
74. http://www.immunise.moh.govt.nz/documents/safetymonitoring-0205.pdf
75. Prime Minister’s Statement, Hansard, Tuesday, 10 February 2004, http://www.clerk.parliament.govt.nz/Content/Hansard/Final/FINAL_2004_02_10.htm


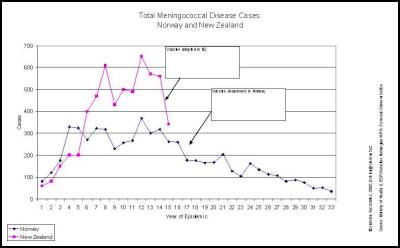
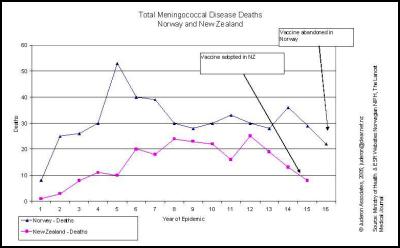
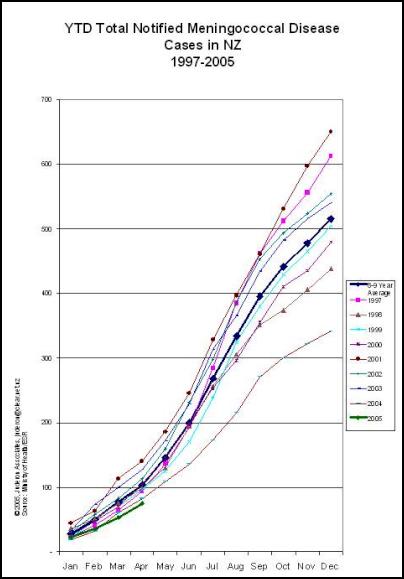

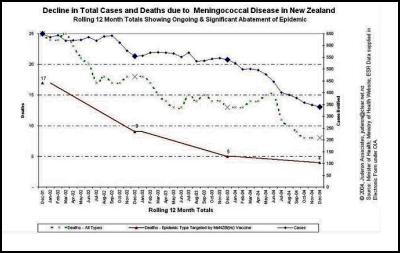
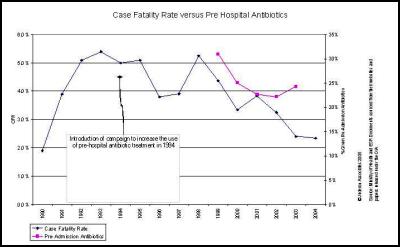
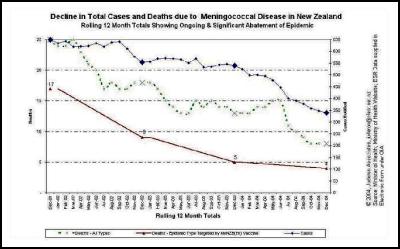
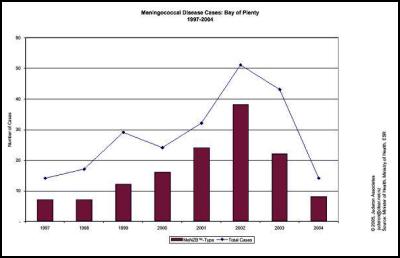
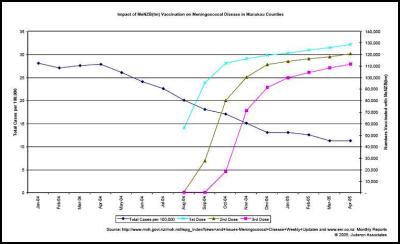
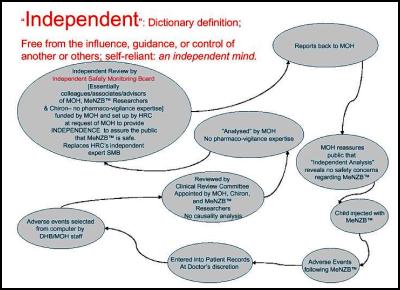
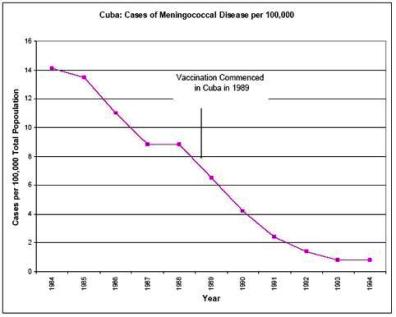
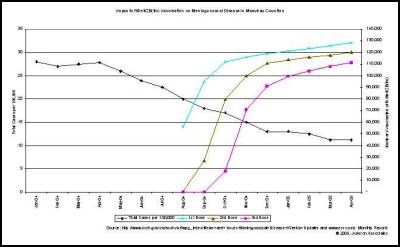
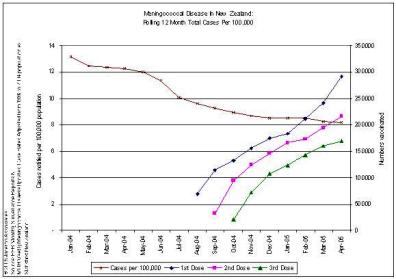
 Richard S. Ehrlich: Deadly Border Feud Between Thailand & Cambodia
Richard S. Ehrlich: Deadly Border Feud Between Thailand & Cambodia Gordon Campbell: On Free Speech And Anti-Semitism
Gordon Campbell: On Free Speech And Anti-Semitism Ian Powell: The Disgrace Of The Hospice Care Funding Scandal
Ian Powell: The Disgrace Of The Hospice Care Funding Scandal Binoy Kampmark: Catching Israel Out - Gaza And The Madleen “Selfie” Protest
Binoy Kampmark: Catching Israel Out - Gaza And The Madleen “Selfie” Protest Ramzy Baroud: Gaza's 'Humanitarian' Façade - A Deceptive Ploy Unravels
Ramzy Baroud: Gaza's 'Humanitarian' Façade - A Deceptive Ploy Unravels Keith Rankin: Remembering New Zealand's Missing Tragedy
Keith Rankin: Remembering New Zealand's Missing Tragedy