Investigation: The Meningococcal Gold Rush
The Meningococcal Gold Rush
By Barbara Sumner Burstyn
& Ron Law
EXECUTIVE SUMMARY: New Zealand's meningococcal disease story, as unravelled through analysis of previously secret documents obtained under the Official Information Act, reveals that the New Zealand government, media and public have been misled and manipulated by officials, advisors and scientists alike.As a result of this manipulation, the government has committed an unprecedented 200 million taxpayer dollars to a mass vaccination experiment of 1.15 million New Zealand children with an untested and experimental vaccine. Despite being reassured by a bevy of pro-vaccine and vaccine manufacturer sponsored experts and none-less than the Minister of Health herself that the MeNZB(tm) vaccine is thoroughly tested and proven to be safe and effective, we reveal that Chiron's MeNZB(tm) vaccine was never used in the trials used to approve its license. We reveal that despite assurances, there is no evidence that the MeNZB(tm) vaccine will actually work as promised.
We believe that the magnitude of policy, regulatory and scientific misconduct is such that not only should vaccination with this vaccine be halted forthwith, but that the meningococcal vaccination program should be independently audited and the circumstances surrounding the development and implementation of the program subjected to a full Royal Commission of Inquiry.
* * * * * * In January 2002 the Minister of Health Annette King announced that “$100 million-plus” had been set aside to fund development and implementation of a vaccine to combat New Zealand’s unique strain-specific meningococcal group B bacterium [1]
By May that year, following Ministry of Health negotiations with the preferred contract supplier, Chiron Corporation, that figure had become “a commitment of up to $200 million.” [2] By September 2004 the sum of $250 million was being mentioned in parliament. [3]
In a July 7 2004 press release Ms King described the development and approval of the MeNZB™ vaccine as ‘fantastic news.’ She went on to explain that the MeNZB™ vaccine had been “specifically developed with scientists from biotechnology company Chiron Corporation.” Cabinet was told in 2001, immediately prior to approving the signing of the Chiron contract, that the deal included the "development of a unique or 'orphan' vaccine." [4]
Chiron’s own press release declared they [Chiron] had specifically developed the vaccine. [5] The company quoted Ms King congratulating them "for their effort and dedication to this project."
But documents received under the Official Information Act reveal that the MeNZB™ vaccine was not developed by Chiron Corporation. It was developed by the Norwegian Institute of Public Health. Chiron had bought the rights to mass manufacture and market the Norwegian meningococcal B vaccines in November 1999, nearly two years before the New Zealand government signed the initial contract with the company. [27]
Last March, in replying to a question in the House from National MP Dr Lynda Scott, the Minister of Health declared that $10.7 million has been spent on the development of the group B meningococcal vaccine. [49] While Cabinet papers, released under the Official Information Act, had most financial details censored as being commercially sensitive, it would appear that of the total $200 million cost, Chiron will net around a cool $140 million for developing and supplying the already developed vaccine. [28]
From the Norwegian perspective, the off-loading of their Norwegian specific vaccine to Chiron must have been a godsend given it had likely invested over a hundred million $US in double blind, placebo controlled studies involving 170,000 people and over 2,000 doctors and nurses for a vaccine that was never licensed for use in mass vaccination. (New Zealand’s preliminary trials involved about 1,500 people and cost at least $7.8 million). In parliament on 19 October 2004 Ms King stated "[the Norwegian Government decision not to approve their vaccine for use] was ... based on the evidence they had, that it did not stack up in terms of cost-benefit, so they did not continue with it."
However The Lancet medical journal reported in 1991 that the Norwegian Institute of Public Health found that the large and robust clinical trials proved the vaccine to have insufficient efficacy to justify its use in a mass vaccination program. [6] The Lancet paper also contained data showing that the epidemic was waning naturally by the completion of the trials. The incidence had declined from peak levels by about 50%, similar to the natural decline that had occurred in New Zealand when the vaccine was approved.
A further press release by Chiron declared, "Since the signing of the initial 2001 agreement with the New Zealand Ministry of Health, Chiron has supplied vaccine for clinical studies conducted in collaboration with the Ministry of Health and the University of Auckland." [7] While they are technically right, the inference in New Zealand has always been that the vaccine for the trials was strain-specific and created expressly by Chiron Corporation to combat New Zealand’s ‘unique’ meningococcal group B bacterium.
Financial and development questions aside, the documents received under the Official Information Act reveal that not only was the vaccine not developed by Chiron, but the vaccine used in the clinical studies in New Zealand was not even made, as claimed, by Chiron. The vaccine used in the studies was both developed and, according to minutes of the MAAC vaccine subcommittee, manufactured by the Norwegian Institute of Public Health.
This raises a serious issue. In answer to another question by National’s Dr Lynda Scott (28 May 2003) the Minister of Health said, “New Zealand will not acquire the intellectual property rights (to the vaccine) because New Zealand does not have manufacturing experience in producing Outer Membrane Vesicle (OMV) vaccines. If this were indeed possible, repeated clinical trials would need to happen in New Zealand for the production of MeNZB. When a vaccine is produced at a new manufacturing site, it is deemed for clinical reasons a new vaccine and would require a full clinical investigation and licence application.” [50]
Therefore, by the Minister’s own standards, Chiron should be conducting new clinical trials on its own MeNZB™ vaccine in New Zealand. After all, the vaccine currently being deployed in this country is not the same as the one used in the trials. It is made by a different organisation, in a different laboratory, and in a different country to that of the trial vaccine. Despite this, Chiron has been given a licence based on a vaccine made by Norway in direct contradiction to the Minister’s own statements.
Of the two trial groups that have been tested with the Chiron produced MeNZB™ vaccine, the minutes of the Minister’s MAAC vaccine sub-committee noted that one involved a total of ten adults. The number of 8-12 year old children involved in the other group is still unknown, but is unlikely to be more than a handful. [10]
The documents also show that when Chiron finally manufactured and tested its MeNZB™ vaccine in its Italian factory, it was so late in the trial process that the Minister’s MAAC Vaccine Sub-Committee notes that no results of either of the tests were completed when they recommended the vaccine be approved for general release.
The expert committee's conclusion was that, “the current data supplied provides very limited data on its effectiveness,” and "evidence of efficacy is not compelling.” They went on to say, “the Committee was concerned that there was no efficacy data for the proposed [MeNZB™] vaccine, and were not convinced that the efficacy and safety monitoring during the roll out was sufficient to maintain public safety and confidence.” [29] The Ministry of Health’s Dr Jane O’Hallahan has admitted that the MeNZB™ vaccine would be rolled out ‘without efficacy data.’
The MAAC vaccine subcommittee noted that not a single study of Chiron manufactured vaccine had been completed. MAAC also declared "there are a number of issues relating to the manufacturing and quality data that are to be addressed by Chiron."
The very next day the Minister approved Chiron's MeNZB™ vaccine. In a press statement announcing the approval the Minister said, “MedSafe is assured that the vaccine is safe and effective given all the information currently available to it.” [9]
One can only marvel at the speed and efficiency of government agencies that are able to resolve such outstanding issues in a single day. This is especially pertinent given that on the same day MedSafe extended by 18 months [30] the about-to-expire use-by date of over 300,000 doses of the vaccine that had been produced in commercial quantities by Chiron, months in advance of the completion of trials and licensing, [37]
To add to the complexity of the issue, at the same time as MAAC was citing manufacturing and quality data issues, regulators in the United States, Britain and Brazil were cognizant of manufacturing and quality problems at Chiron’s plant in the UK, and the same Italian plant used to manufacture the MeNZB™ vaccine. Subsequently British regulators cancelled Chiron’s UK factory license due to breaches of ‘Good Manufacturing Practice’ resulting in 50 million doses of flu vaccine being dumped in August. At this time Chiron has been unable to rectify the manufacturing problems.
As has been pointed out by the Ministry the MeNZB™ was not made in the Liverpool factory. It was made in Chiron’s Italian factory. However, this same factory produced the more than four million doses of MMR vaccine deployed in Brazil, that were recalled due to several hundred serious adverse reactions, including anaphylaxis. [43]
To put the Ministry’s attitude to Chiron into perspective it may be useful to recall the situation surrounding the Minister’s response in 2003 to complementary medicines made by Pan Laboratory. Pan breached Good Manufacturing Practice in the case of a pharmaceutical product that caused serious adverse effects. Three months later, this resulted in the mandatory recall and destruction of over 1,600 unaffected supplements at a cost to industry of some $400 million in Australia and New Zealand. Industry sources reveal that one New Zealand company lost 20 million dollars alone due the New Zealand Minister’s recall, despite having independent analysis proving that their product was up to standard. The Minister’s response was so definitive that she ordered the destruction of all recalled goods.
Further documents received under the Official Information Act reveal that while the country has been sold on the need for three vaccinations to bring any immune response up to a suitable level, the Ministry’s own unpublished cost benefit analysis was based on five doses. [11]
Referred to by the Minister as an, ‘independent economic evaluation of the anticipated economic benefits [of the vaccine]” [38] the analysis was undertaken by the faculty that stood to gain many millions of dollars of research funding from Cabinet approval of the vaccination program. The authors included senior meningococcal vaccine researchers and their colleagues at Auckland University. Puzzlingly, neither the report [nor the Minister] disclosed this important fact to Cabinet; the report falsely declared, “Competing Interests: None.”
Another cost benefit analysis by Treasury in 2001 showed that the cost-to-benefit ratios were seven times those normally used by Pharmac to approve funding of prescription medicines [12] and that was before the significant declines in disease and deaths that have occurred naturally.
An Honours student at Canterbury University also did a cost benefit analysis. Whilst presented at the New Zealand Association of Economists conference in Wellington in June 2004, [45] the paper has not been posted on the website as is usual practice but has been ‘temporarily withdrawn’ from public purview. This is considered unusual as the Audit Office says the paper is in public domain once presented. The paper is said to have revealed that the MeNZB™ vaccination program did not stack up economically and, like the Auckland District Health Board, questioned the program’s rationale. [46] A university source has revealed that the paper was removed to protect the interests of the student after the University received a threatening letter ‘advising against publication.’ We are aware of the student’s name and have been asked not to make it public as to do so could jeopardize their career options. We are also informed that the student was approached by officials from other government departments and congratulated for raising questions they were not allowed to.
Other MOH accounting is also dubious. The need for a new strain-specific vaccine was based on 5,000 plus cases of epidemic strain meningococcal B. This number has appeared in published scientific abstracts at conferences including San Francisco, Milwaukee, Boston, Mexico and Auckland. But source documents show that less than 50 percent of ‘total’ cases have been confirmed as being of the epidemic meningococcal B strain targeted by the MeNZB™ vaccine.
The 5,000 plus figure includes cases caused by at least six other strains of meningococcal bacteria, as well as unconfirmed ‘suspected’ cases. Even this figure is in question as a MOH commissioned report suggests that between 10 and 25 percent of notified cases are likely to have been falsely diagnosed. [31] Nevertheless, Cabinet was falsely told that, "the current epidemic has been caused by a single strain of group B meningococcal bacterium." [14]
The World Health Organisation states that if the death rate for meningococcal disease is less than 5 percent then it is likely that cases have been over-diagnosed. [32] The death rate in New Zealand in 2003 and 2004 has been 2.3 percent for all types combined. Data from the Minister shows the epidemic strain targeted by the MeNZB™ vaccine has averaged a death rate of just 1.4 percent for the past two years.
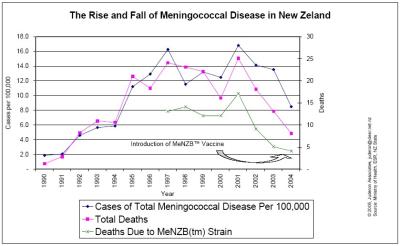
There are other anomalies between the Minister’s figures and those reported to Cabinet. Cabinet was told that meningococcal disease would kill 20 New Zealanders per year for the next ten years, and that the MeNZB™ vaccine would avert 13.6 deaths per year. Yet since 2001, total deaths for all age groups have declined naturally by about 70 percent while case numbers have dropped by 48 percent. [13] Deaths due to the strain of bacteria targeted by the MeNZB™ vaccine have declined by 76% since peak levels in 2001. A look at death rates across all strains of the disease show a natural decline in the disease burden and follow somewhat the disease cycle decline experienced in Norway, Cuba, Ireland and numerous other countries. [16] [51 (Graph One)] In short, the total case numbers are at their lowest levels since 1994, and death rates due to meningococcal disease are at their lowest levels since 1991, the year the 14-year epidemic began. Puzzlingly, in the 7 July 2004 press statement Ms King said, “The epidemic has shown no signs of abating.”
The Minister has refused to release age related deaths for the epidemic strain. But our best estimates are that the vaccine, if it proves effective, will prevent at most 1 or 2 deaths per year in under 20 year olds out of approximately 700 who die each year from all causes. In other words 0.2% of all deaths in the under 20’s might be prevented. Put another way, if the government applied the same cost-benefit analysis to preventing the other 99.8% of deaths, it would be spending over $100 billion.
The numbers game extends to international medical conferences. At the Chiron sponsored, Seventh Annual Conference on Vaccine Research in Virginia, USA in May 2004, the MOH meningococcal vaccine program director, Dr Jane O‘Hallahan was an invited speaker. Her abstract reads in part, “With the epidemic claiming up to one life every two weeks in a nation of four million people, this collaborative group is working against the clock.” [21]
At the time the abstract would have been written, the death rate had fallen to one death every four weeks from all forms of meningococcal disease and one death every three months from the epidemic strain of bacteria
In another anomaly, in 2001 the Ministry of Health told Cabinet [33], through a document requesting funding for the Meningococcal B vaccine, that the vaccine would most likely cause herd immunity. "[The preferred] option also has the benefit of likely herd immunity. This is expected but cannot be quantified." The document contains other allusions to expectation of herd immunity. Later in the document the Minister's Office memo categorically stated that herd immunity had been achieved in meningococcal vaccination programs in the United Kingdom and Cuba.
But the United Kingdom program used a Meningococcal C vaccine, and the Cuban study used a B/C combo, vaccines that are totally different in their make-up. Meningococcal B vaccines cannot be made using the same process as meningococcal C vaccines as they would induce antibodies that attack the brain. It is generally understood to be scientific fraud to compare different entities as if they were the same.
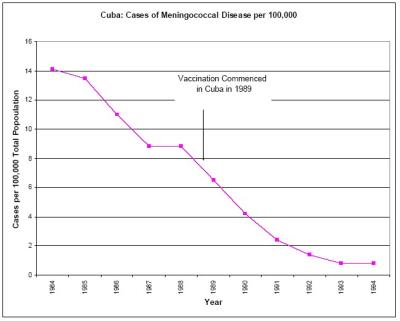
There is also no compelling scientific evidence that the Cuban vaccination program conferred herd immunity. Reference to herd immunity in the medical literature is predicated on no natural decline in case numbers pre and post vaccination, and that the entire decline is due to the vaccine, and that the vaccine reduces carriage and spread of the bacteria. The incidence of meningococcal B was in steep natural decline at the time the Cuban immunization program began. [34, see Graph Two] We can find no credible evidence in the scientific literature that suggests that Meningococcal B vaccines reduce the carriage and spread of the bacteria and hence have a herd effect.
It is also of interest that the MeNZB™ researchers themselves now acknowledge that there is no body of evidence that MeNZB™-type vaccines confer herd immunity. [35] Outer membrane vaccines such as the MeNZB™ vaccine do not reduce carriage or the spread of meningococcal bacteria. The only grounds for raising the prospect of herd immunity by the officials would appear to be to amplify the potential benefits of the meningococcal vaccine in an attempt to convince Cabinet that Treasury had been too conservative in their assessments. [52 (Graph Two)]
Perhaps a paper given by the Ministry of Health’s Dr O'Hallahan at a meningococcal disease conference in Norway in 2002 goes some way to explaining this type of pressure. Entitled, "How to harness the political will and implement an OMV vaccine solution to combat a devastating epidemic,” the paper appears to have outlined ways to convince a government to become involved in funding a new vaccine. [44] Another blatant example of the use of the ‘harnessing of political will’ can be seen in the 2001 Cabinet papers when Cabinet gave approval for the $200 million project. Paragraph 87, the last paragraph before the recommendations section, simply states, “Large public and media reaction is expected if campaign does not go ahead.”
The choice of Chiron Corporation as the exclusive manufacturer of the New Zealand vaccine also raises a number of questions. Cabinet papers show that Ministry officials rejected competitor options and entered into the initial contract with Chiron knowing that Chiron would only produce the vaccine if they got the contracts to both manage the trials and supply the vaccine for the clinical trials and the roll-out. [17] This raises conflict of interest issues as Chiron effectively controlled weather or not the government paid it for 3-4 million doses of its vaccine.
Papers released under the Official Information Act reveal that the Ministry of Health and its advisors discarded a competitor of Chiron's from consideration as a supplier of a meningococcal B vaccine because their vaccine was a B/C combo. This rejected supplier manufactured the Cuban vaccine. Having discarded a competitive supplier because the vaccine was a combo, the Ministry of Health and Chiron then used that discarded competitor’s efficacy data and the alleged herd effect from the Cuban studies of the discarded vaccine to justify the licensing and use of Chiron’s untested MeNZB™ vaccine in New Zealand. [17]
Having apparently already acquired the rights to the Norwegian meningococcal B vaccines, combined with the Ministry’s discarding of a key competitor, Chiron appears to have held all the cards.
Paradoxically, Chiron's publicly stated intentions are to produce a combined meningococcal B and C vaccine. Government papers show that trials have been undertaken in New Zealand using Chiron's meningococcal C vaccine Menjugate™ combined with the Norwegian vaccine.
Chiron’s Menjugate™ vaccine was also used as the control in a MeNZB™ trial of infant’s in New Zealand. The infants were given Menjugate™ vaccine as a so-called ‘placebo.’ [18] This fact is not mentioned in the MAAC minutes but was disclosed in a paper presented by Chiron at a scientific conference in Japan in October 2004.
This raises serious questions regarding informed consent. Were parents and guardians aware that Chiron was undertaking what would appear to be unapproved trials of a vaccine that is not licensed for use in New Zealand nor the USA?
In October 2004 Chiron stated that the FDA had requested further information regarding its application to license Menjugate™ in the USA. [19] Since then, Chiron has withdrawn their USA application for approval of Menjugate™ on the premise that they want to introduce a combo vaccine with a broader market appeal. This seems to be a puzzling move, however, given that they had recently completed phase III clinical trials on Menjugate™; it seems odd that if the trial results had been positive that Chiron would not have proceeded with its application.
So, what are Chiron’s motives? Aside from simple commercial opportunism, there is wide market appeal for a New Zealand financed and trialed vaccine. New Zealand’s Environmental Science and Research (ESR) annual report this year commented, 'the trials are definitely of international interest because the same strain is now causing problems in Europe, although not yet at an epidemic level … It’s of huge international interest.” [22] Chiron’s competitor Aventis said recently that meningitis B must be the next vaccine target in the US. [3].
Secondly as the meningococcal B vaccine gold rush gains momentum there is significant competitive pressure to gain dominance in this field. The WHO noted in a report on the state of the art of vaccines, that research institutes have formed alliances with pharmaceutical companies. The Cuban Finlay Institute and GlaxoSmithKline, Norwegian Institute of Public Health and Chiron, and the Dutch RIVM and Wyeth have formed alliances. Other companies such as Microscience (USA) are also researching novel meningococcal B vaccines, as is Aventis. [23]
Within days of Chiron’s MeNZB™ vaccine being approved in New Zealand, the U.S. Treasury Department granted GlaxoSmithKline a first-of-its-kind license to market the Cuban vaccine against meningococcal B bacteria. [24] Interestingly, Chiron has licensed its competitor, Aventis, to market its Menjugate™ vaccine in Europe.
Despite all of this, pharmaceutical companies themselves acknowledge that the meningococcal B bacterium is uniquely resistant to vaccination. [25] In fact in 2000 Chiron’s Dr Rappuoli stated that, "Conventional research approaches to develop effective vaccines against different strains of group B meningococcus have failed." In 2002 Dr Rappuoli reported that despite years of effort, biomedical scientists failed to find a protective molecule that would induce immunity to type B meningococcal disease. [39]
Perhaps this is why Chiron has recently commenced clinical trials of a genetically engineered broad-spectrum meningococcal B vaccine. This begs the question; is New Zealand’s foray into the international vaccine game little more than a form of naive and cynical political manipulation.
From Chiron’s perspective, given the recent comment by Merrill Lynch that “with only one of Chiron's three businesses doing well” growth was likely to slow, the $140 million it is being paid for “developing and trialling” a vaccine made by someone else, then being handed a license to supply 3.5 million doses to a guaranteed market must seem convenient. Especially given they have been spared the expense of Phase III trials.
While Chiron’s withdrawn Menjugate™ made it to Phase III trials [48] the New Zealand Minister vetoed the idea of Phase III trials for MeNZB™ saying it was agreed that the amount of safety and efficacy information on the Norwegian specific vaccine produced in Norway for Norway could be bridged to the New Zealand clinical trials.
Phase III trials are generally considered to be the place where the true effectiveness and safety of a vaccine becomes known. The Minister added that additional trials would have added little value given the comparable information already available. She went onto say this approach allowed the rapid introduction of the vaccine. [40]
This tactic is akin to Vioxx being approved based on Celebrex data; that because there was only a minor difference in the two drugs, the adverse effects and benefits would be the same. The fact that the Norwegian vaccine designed for their epidemic was never approved for routine use in its own country because it wasn’t effective enough seems to have slipped from memory here.
It should also be noted that it was only through extensive phase III testing that it was proven that Vioxx and Celebrex are lethal drugs responsible for tens of thousands of deaths in the US and several hundred at least in Australasia. Is the reason for the Minister’s dismissal of Phase III trialing because such testing might have undermined the political and public momentum for the MeNZB™ vaccination program?
While Chiron’s motives may be transparent, it is the roles of New Zealand researchers and medical regulators that are of primary concern.
Numerous countries are beginning to ask about the alliances between science, research, regulators and the pharmaceutical industry and the conflicts of interest those alliances create. Specifically there are significant connections and conflicts of interest between the corporation holding the trademark on the MeNZB™ vaccine and researchers and regulators in New Zealand. The fact that the government gave away ownership of the intellectual property is one mystery. The Chiron funded conference mentioned earlier is a prime example of how one medical professional is building a reputation and career courtesy of a company whose product she is meant to be trialing in an unbiased way.
Potentially dangerous conflicts of interest extend to the MeNZB™ adverse event monitoring system. This system is overseen by hand picked pro-vaccine specialists. In two cases these specialists are colleagues of the meningococcal B researchers. This ‘independent’ monitoring board has developed a method that only considers known adverse events, discounting deaths following meningococcal vaccination as due to ‘accident’ or ‘other unrelated illness’. It should be noted that the Minister has not denied the two deaths reported to have occurred during the trials. [42] They were dismissed as being not relevant to the vaccination trial. This is at odds with good pharmaco-vigilance practice.
There is the question of why the appointed champion of the meningococcal B vaccine program is also on the MAAC vaccine sub-committee that recommended the approval of the vaccine – in spite of concerns about manufacturing and quality of the vaccine and lack of evidence of efficacy. This final MAAC meeting was convened with less than a days notice. The majority of members had not seen the clinical data so the champion of the meningococcal B vaccine program then participated in the final full MAAC meeting to brief the experts before they made their decision. The minutes note that this extensive input ‘had no influence on the final decision.’
The minutes of a second MAAC vaccine subcommittee meeting held the day before the final full MAAC meeting in July are apparently so contentious that the Minister has refused to release them under the Official Information Act stating that to do so would discourage full and frank discussions. Interestingly, no mention is made of this meeting in the final MAAC minutes.
Then there are the ethical questions surrounding the Ministry of Health downloading school rolls into its new National Immunisation Register to capture all children’s ID information. Was informed consent granted for that? [26]
The questions surrounding the MeNZB™ vaccine continue to mount. Given that government documents reveal that it was a vaccine made in Norway and not Chiron's Italian made MeNZB™ vaccine that was used in the New Zealand trials, and given that a virtually un-tested vaccine rolled out ‘without efficacy data” is now in general use, the primary question may be: is the Chiron MeNZB™ vaccine now being used in the current mass vaccination of 1.15 million young New Zealanders itself an uncontrolled medical experiment?
On December 7 2004 Merrill Lynch said that Chiron Corporation “may become the vaccine supplier of last resort, given its manufacturing problems and tarnished reputation.” [47] With hind-sight, maybe it already has.
Perhaps a recent comment in the New York Times goes some way to explaining the ideology behind it all. In a debate before the US Advisory Committee on Immunization Practices an immunization expert, discussing the introduction of a new meningococcal vaccine said, “Frightening parents about the consequences of failing to vaccinate their children will most likely be part of the campaign. For that task, meningococcal meningitis is ideal.”
In New Zealand the Ministry of Health has done more than frighten the public. They appear to have participated in a new orchestrated litany of lies and a massive breach of the public trust.
********* FOOTNOTES:
The Meningococcal Gold Rush: References
Barbara Sumner Burstyn, Ron Law
February 2005
1. http://www.beehive.govt.nz/ViewDocument.cfm?DocumentID=129062. http://www.beehive.govt.nz/ViewDocument.cfm?DocumentID=14056
3. http://www.clerk.parliament.govt.nz/Content/Hansard/Final/ FINAL_2004_09_15.htm#_Toc84652997
4. Memorandum to Cabinet Health and Education Committee: Request for Group B Meningococcal Vaccination Campaign Funding. Office of the Minister of Health, 2001, Para 3 –
5. http://www.chironvaccines.com/company/ vaccines_Press_Area_7_July_2004.php
6. Lancet. 1991 Nov 2;338(8775):1093-6
7. http://www.chiron.com/investors/pressreleases/press_release070704.html, July 8 2004
8. MAAC VSC Minutes, 5 April 2004
9. MAAC Minutes, 6 July 2004
10. MACC VSC Minutes, 5 April 2004
11. An Economioc Evaluation of Vaccination against Meningococcal Disease, 2001, Milne et al
12. Cabinet Memorandum, December 2001
13. Minister of Health, Answers to Parliamentary Question 16087 (2004) and PQ 16985 (2004).
14. Cabinet Memorandum, December 2001)
15. The Minister provided figures in answer to PQ 16087 (2004) that deaths from the targeted strain had dropped from a high of 17 in 2001 to 4 in 2003. ESR figures provided under the OIA said there were 5 deaths in 2003. Estimates are that there have been 4 deaths for all ages due to the targeted strain in 2004.
16. MOH website, and answers to PQ 16087 (2004) Deaths due to all strains of the disease declined to 13 in 2003 and 8 in 2004 for all ages. In 2003 and 2004 there have been 5 and 4 deaths respectively due to the epidemic strain of meningococcal B.
17. Cabinet Memorandum, December 2001
18. Trial V60P50
19. http://www.chironvaccines.com/company/vaccines_Press_Area_20_October_2004.php
21. http://www.nfid.org/conferences/vaccine04/abstracts.pdf
22. MOH, (2004) The Epidemiology of Meningococcal Disease in New Zealand
23. State of the Art of New Vaccines: Research & Development Initiative for Vaccine Research, World Health Organization, Geneva, April 2003
24. http://www.medicc.org/Medicc%20Review/I/varied/html/health_news_from_cuba.html
25. “Chiron Vaccine is building on its technical expertise in developing the meningococcal vaccines to develop a recombinant vaccine against serogroup B. Sequence variation of surface-exposed proteins and cross-reactivity of the serogroup B capsular polysaccharide with human tissues have hampered efforts to develop a successful vaccine.” Quote from Chiron’s website, http://www.chironvaccines.com/company/vaccines_Pipeline_Men_B.php
26. Quote from MeNZB Update the majority of our schools “have now received upgrades to their own school roll systems that will enable a '1' button push to produce the required roll extracts for the upcoming campaign.” http://www.imac.auckland.ac.nz/new/meningococcal/menzbge04_04.pdf
http://www.simpl.co.nz/News/Press/PressRel_2.aspx
http://www.moh.govt.nz/moh.nsf/0/e2fcda97a841bdb2cc256e9b000a3264?OpenDocument27. [Chiron to Co-Develop Combination Meningitis B/C Vaccine With the Norwegian Institutes of Public Health; Company Gains Access to Meningitis B Vaccine, Nov 1999, http://stg.syndnet.thomsonfn.com/InvestorRelations/PubNewsStory.aspx?partner=5425&storyId=74296]
28. http://www.chiron.com/investors/shareholder/2003_10K.htm
29. Ref: Minutes MAAC VSC 5 April 2004
30. http://www.nzherald.co.nz/index.cfm?ObjectID=3577033
32. http://www.who.int/emc-documents/meningitis/docs/whoemcbac983.pdf
33. Memorandum to Cabinet Dec 2001
34. (Rodríguez, AP. et al, .Impact of Antimeningococcal B Vaccination • Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 94(4), Jul./Aug. 1999).
35. Proceedings of the Meningococcal Vaccine Strategy World Health Organization Satellite Meeting, 10 March 2004, Auckland NZ; published in NZMJ, Aug 2004.
37. Chiron Press Release, January 2004
38. Ref: Answer to PQ 17136 (2004). Rt Hon Winston Peters to the Minister of Health (24 November 2004
39. Moxon and Rappuoli, 2002
40. 17975 (2004) Published - Health - Normal Reply Question: Have any Phrase III Clinical studies been undertaken on the MeNZB (tm) vaccine? If so, when and what were the results?
Portfolio: Health. Minister: Hon Annette King, Date Lodged:09/12/200441. http://www.medsafe.govt.nz/Profs/adverse/Minutes111.htm
42. MOH website – ISMB report, October 2004
43. New York Times, 28 Aug 2004; http://query.nytimes.com/gst/abstract.html?res=F10812FE355A0C7B8EDDA10894DC404482 and later reports.
44. O’HALLAHAN, J. How to harness the political will and implement an OMV vaccine solution to combat a devastating epidemic Meningococcal Vaccine Strategy, Ministry of Health, Auckland, New Zealand. Thirteenth International Pathogenic Neisseria Conference held at the Norwegian Institute of Public Health, Oslo, Norway; Sept. 1-6, 2002
45. The New Zealand Association of Economists (Inc) 45th annual conference, 2004 Programme, 30 June – 2 July 2004. Eradicating meningococcal disease in New Zealand: Is it worth it? To whom? And who decides? Name of author withheld.
46. Letter dated 24 June 2002, on file
47. http://www.forbes.com/markets/2004/12/02/1202automarketscan18.html
48. http://www.fdanews.com/cgi-bin/udt/im.display.printable?client.id=wbi-dpa&story.id=32122
49. PQ 2100 (2004). Dr Lynda Scott to the Minister of Health (9 March 2004): How much money has been spent on meningitis programmes and where has this money been spent, specified by programmes and allocated funding?
Hon Annette King (Minister of Health) replied: Between 1 December 1997 and 31 December 2003, a total of $21.7 million (GST inclusive) has been spent on the Meningococcal Vaccine Strategy. Of this, $10.7 million has been spent on the development of the group B meningococcal vaccine, $7.8 million on conducting clinical trials, and $0.7 million on planning and preparation for the national immunisation rollout programme. A further $2.5 million has been spent on operational funding to support what will be New ZealandÆs largest immunisation programme.
50. PQ 5241 (2003). Dr Lynda Scott to the Minister of Health (28 May 2003):
Will New Zealand acquire the intellectual property rights to the meningococcal
vaccine that is being trialled?Hon Annette King (Minister of Health) replied: No. New Zealand will not acquire the intellectual property rights because New Zealand does not have manufacturing experience in producing Outer Membrane Vesicle (OMV) vaccines. If this were indeed possible, repeated clinical trials would need to happen in New Zealand for the production of MeNZB. When a vaccine is produced at a new manufacturing site, it is deemed for clinical reasons a new vaccine and would require a full clinical investigation and licence application.
51. Graph One – The Rise And Fall Of Meningococcal Disease In NZ
Click for big version52. Graph Two – The Experience In Cuba
Click for big version************* Barbara Sumner Burstyn is a free-lance writer based in the Hawkes Bay. She is interested in issues of accountability.
Ron Law's career spans twenty years as a medical laboratory scientist, including 10 years as a clinical biochemistry lecturer, 5 years as a university business management lecturer, including research methodology, 4 years as executive director of a trade association, and more recently as an independent risk & policy analyst. Ron has a masters degree in international business studies and an applied theology degree. Ron was appointed by the Ministry of Health as a member of the expert group that advised the Director General of Health on the reporting and management of medical injury in New Zealand's healthcare system.
Seen feedback to: Ron Law and/or Barbara Sumner Burstyn
© Barbara Sumner Burstyn, Ron Law February 05


 Martin LeFevre - Meditations: Regarding Popes, Dopes And Hopes
Martin LeFevre - Meditations: Regarding Popes, Dopes And Hopes Binoy Kampmark: Fantasy And Exploitation | The US-Ukraine Minerals Deal
Binoy Kampmark: Fantasy And Exploitation | The US-Ukraine Minerals Deal Gordon Campbell: On The Aussie Election Finale
Gordon Campbell: On The Aussie Election Finale Martin LeFevre - Meditations: The Enlightenment Is Dead; What Is True Enlightenment?
Martin LeFevre - Meditations: The Enlightenment Is Dead; What Is True Enlightenment? Ian Powell: Widening Gap Between Health System Leadership And Health Workforce
Ian Powell: Widening Gap Between Health System Leadership And Health Workforce Keith Rankin: The Great World War 1914-1945 - Germany, Russia, Ukraine
Keith Rankin: The Great World War 1914-1945 - Germany, Russia, Ukraine