NTCELL® Continues to Reverse the Progression of Parkinson’s
NTCELL® Continues to Reverse the Progression of Parkinson’s Disease – One Year After Implant
27 January 2016 – Sydney, Australia & Auckland, New Zealand – Over a year after treatment all four patients who took part in Living Cell Technologies Limited’s Phase I/IIa clinical study of NTCELL® for Parkinson’s disease remain well, and at 58 weeks post-implant there are no safety concerns.
In all four patients NTCELL treatment has stopped the progression of Parkinson’s disease as measured by globally accepted and validated neurological rating scales.
Moreover, as the chart below shows, after 58 weeks there is a clinically and statistically significant improvement in all the patients’ neurological score from their pre-implant baseline.
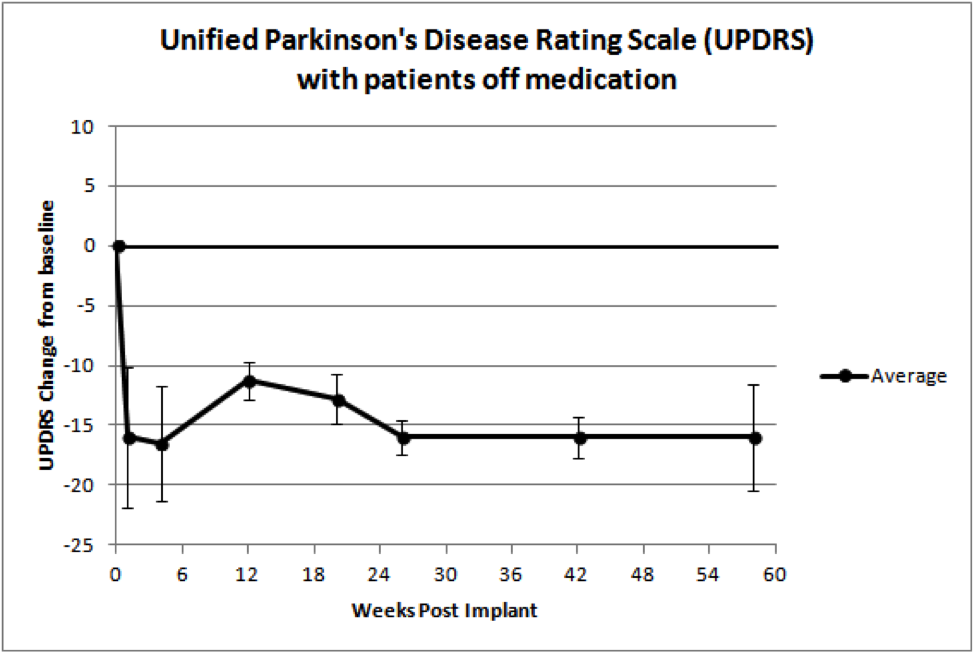
NTCELL’s ability to decrease UPDRS by an average of 16 points after 58 weeks is clinically significant, representing a 3 to 4 year reversal of neurological deterioration. In the first patient the improvement is maintained at 74 weeks after NTCELL implant.
Dr Ken Taylor, chief executive, says the sustained improvement of the patients is pleasing, particularly as no other treatment has been able to maintain long term reversal of the effects of Parkinson’s disease.
“We are delighted with the continued positive outcome of the study. It certainly adds anticipation and motivation to our authorised Phase IIb study, which we plan to initiate on 24 February.”
The planned Phase IIb study aims to confirm the most effective dose of NTCELL, define any placebo component of the response and further identify the initial target Parkinson’s disease patient sub group.
“Our goal is to obtain provisional consent and launch NTCELL as the first disease modifying treatment for Parkinson’s disease in 2017,” says Dr Taylor.
ENDS


 Hospice NZ: Sustainable Funding For Hospices
Hospice NZ: Sustainable Funding For Hospices David Hill - LDR: Surge In Young Parents’ College Enrolments
David Hill - LDR: Surge In Young Parents’ College Enrolments NZEI Te Riu Roa: School Lunches - Give Schools Option To Use In-School And Community Providers Immediately, Union Says
NZEI Te Riu Roa: School Lunches - Give Schools Option To Use In-School And Community Providers Immediately, Union Says University of Auckland: Billion-dollar Business Lessons From Kiwi Entrepreneur
University of Auckland: Billion-dollar Business Lessons From Kiwi Entrepreneur NZ Veterinary Association: NZVA Celebrates Welcome Additional Support From Veterinary Nurses
NZ Veterinary Association: NZVA Celebrates Welcome Additional Support From Veterinary Nurses Michael King Writers Centre: New Zealand’s National Writer-Residency Organisation Announces Its 2025 International Residency With Australia
Michael King Writers Centre: New Zealand’s National Writer-Residency Organisation Announces Its 2025 International Residency With Australia